2022-10-03 オークリッジ国立研究所(ORNL)
実験では、塩化チオニルを使って、溶融塩から腐食の原因となる不純物を除去している。塩化チオニルを使用しないと、塩がパイプや貯蔵タンクを腐食させるからだ。
研究チームは、太陽熱エネルギー貯蔵用に検討されている豊富な鉱石である市販のカルナライトを溶かし、塩化チオニルを飽和させた不活性ガスに接触させて反応させた。塩の温度を測定し、赤外分光法で放出されるガスを分析することで、反応状況を監視した。
<関連情報>
- https://www.ornl.gov/news/reducing-molten-salts-corrosive-effect
- https://www.frontiersin.org/articles/10.3389/fceng.2022.811513/full
塩化チオニル蒸気との反応による酸素、酸素含有化合物、水酸化物の除去による塩化物塩の精製法
Chloride Salt Purification by Reaction With Thionyl Chloride Vapors to Remove Oxygen, Oxygenated Compounds, and Hydroxides
Joanna McFarlane, Guillermo D. Del Cul, Jordan R. Massengale, Richard T. Mayes, Kevin R. Robb and Dino Sulejmanovic
Frontiers of Chemical Engineering Published:25 April 2022
DOI:https://doi.org/10.3389/fceng.2022.811513
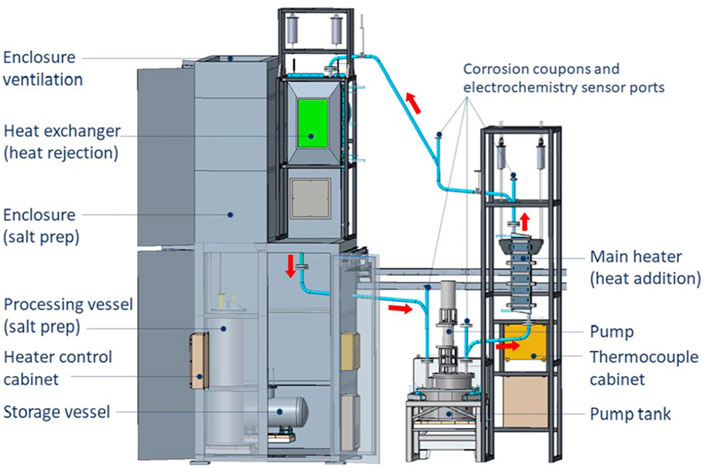
Molten chloride salts (including MgCl2, KCl, NaCl, and ZnCl2) are being considered for heat transfer media for renewable (solar) and nuclear power generators, as fuel carrier for nuclear reactors, and as thermal energy storage media. Impurities such as oxygen, hydroxides, moisture, and sulfur are known to negatively influence the corrosion of materials in contact with the salt (e.g., structural metals). Commercially available chloride salts come with a range of impurities. Before using the chloride salts at high temperature, it is desirable to remove the impurities to increase the performance of the salt and reduce corrosion. In this study, we tested the use of thionyl chloride vaporized into a stream of argon to react with oxygenated impurities in a mixture of MgCl2-KCl-NaCl, removing them as HCl and SO2. The reagent was bubbled through the salt when both above and below the melting point. The reaction was followed using thermocouple data from the salt and by Fourier transform infrared (FTIR) spectroscopy on the exhaust of the reactor. The reaction kinetics were followed by comparing the peaks from SO2 product to SOCl2 reagent in the FTIR spectra. The purity of the salt was assessed at the end of the purification process by x-ray diffraction and inductively coupled plasma analysis. Although the process was effective in removing the oxygen content of the mixture, ternary compounds were formed in the process, including KNiCl3 and KMgCl3. The nickel in KNiCl3 came from the reaction between the salt and the nickel vessel. Thus, these experiments suggest that improvements to the process must be made before using SOCl2 vapors for the purification of chloride salts.



