2022-06-06 オーストラリア連邦研究会議(ARC)
液体ガリウムと組み合わせることで、必要なプラチナの量は、この貴重な金属の地球上の埋蔵量を大幅に増やすのに十分な量となり、CO2削減、肥料製造におけるアンモニア合成、グリーン燃料電池の製造など、化学産業における多くの用途で、より持続可能な解決策となる可能性があります。
プラチナに焦点を当てた今回の発見は、この触媒システムの可能性を示す液体金属の海の一滴に過ぎない。この方法を発展させれば、1,000種類以上の反応に対応する1,000種類以上の元素の組み合わせが可能になります。
この成果は、『Nature Chemistry』誌に掲載された。
<関連情報>
- https://excitonscience.com/news/liquid-platinum-room-temperature-cool-catalyst-sustainable-revolution-industrial-chemistry
- https://www.nature.com/articles/s41557-022-00965-6
低温液体プラチナ触媒 Low-temperature liquid platinum catalyst
Md. Arifur Rahim,Jianbo Tang,Andrew J. Christofferson,Priyank V. Kumar,Nastaran Meftahi,Franco Centurion,Zhenbang Cao,Junma Tang,Mahroo Baharfar,Mohannad Mayyas,Francois-Marie Allioux,Pramod Koshy,Torben Daeneke,Christopher F. McConville,Richard B. Kaner,Salvy P. Russo & Kourosh Kalantar-Zadeh
Nature Chemistry Published:06 June 2022
DOI:https://doi.org/10.1038/s41557-022-00965-6
Abstract
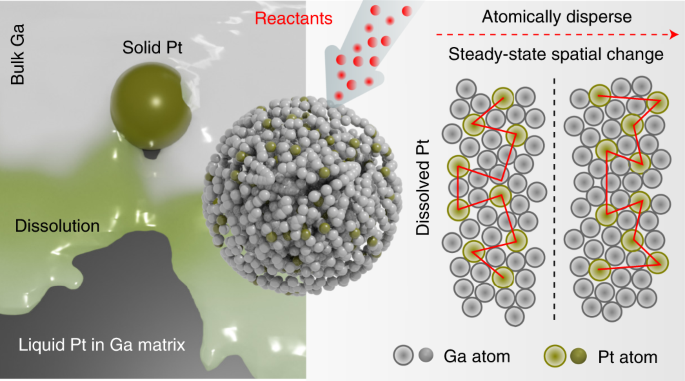
Insights into metal–matrix interactions in atomically dispersed catalytic systems are necessary to exploit the true catalytic activity of isolated metal atoms. Distinct from catalytic atoms spatially separated but immobile in a solid matrix, here we demonstrate that a trace amount of platinum naturally dissolved in liquid gallium can drive a range of catalytic reactions with enhanced kinetics at low temperature (318 to 343 K). Molecular simulations provide evidence that the platinum atoms remain in a liquid state in the gallium matrix without atomic segregation and activate the surrounding gallium atoms for catalysis. When used for electrochemical methanol oxidation, the surface platinum atoms in the gallium–platinum system exhibit an activity of ∼2.8×107mAmg−1Pt,<?XML:NAMESPACE PREFIX = “[default] http://www.w3.org/1998/Math/MathML” NS = “http://www.w3.org/1998/Math/MathML” />∼2.8×107mAmgPt−1, three orders of magnitude higher than existing solid platinum catalysts. Such a liquid catalyst system, with a dynamic interface, sets a foundation for future exploration of high-throughput catalysis.