2022-05-16 アメリカ・ローレンスリバモア国立研究所(LLNL)
何十億年もの進化を経た自然界に存在する最も効果的な分子であっても、自然界以外の用途に改良することは可能である。研究チームは、コンピュータのハードディスクや磁石など数多くの製品に使われている天然元素であるランタノイドに対して最も強力なタンパク質(ランモジュリン)を生物工学的に改良し、アクチニド元素に対する選択性をさらに高めました。アクチノイドは、核廃棄物に含まれるウラン、プルトニウム、アメリシウムなどの放射性金属である。
この研究成果は、『Chemical Science』誌に掲載されました。この研究成果は、天然化合物が環境中の放射性廃棄物とどのように相互作用するかの理解を深めるとともに、特定の放射性金属を捕捉・検出するための新しい分子の開発につながる可能性があります。
<関連情報>
- https://www.llnl.gov/news/going-beyond-mother-natures-molecules-target-radioactive-metals
- https://pubs.rsc.org/en/content/articlelanding/2022/sc/d2sc01261h
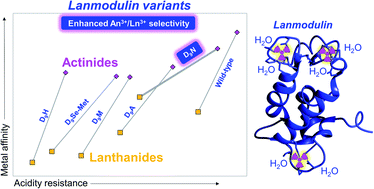
ランモジュリンのランタノイドに対するアクチノイド選択性を、溶媒の配位と第二球の相互作用の制御により工学的に解明。 Engineering lanmodulin’s selectivity for actinides over lanthanides by controlling solvent coordination and second-sphere interactions
Joseph A. Mattocks, Joseph A. Cotruvo, Jr and Gauthier J.-P. Deblonde
Chemical Science Published:26 Apr 2022
DOI:https://doi.org/10.1039/D2SC01261H
Abstract
Developing chelators that combine high affinity and selectivity for lanthanides and/or actinides is paramount for numerous industries, including rare earths mining, nuclear waste management, and cancer medicine. In particular, achieving selectivity between actinides and lanthanides is notoriously difficult. The protein lanmodulin (LanM) is one of Nature’s most selective chelators for trivalent actinides and lanthanides. However, mechanistic understanding of LanM’s affinity and selectivity for f-elements remains limited. In order to decipher, and possibly improve, the features of LanM’s metal-binding sites that contribute to this actinide/lanthanide selectivity, we characterized five LanM variants, substituting the aspartate residue at the 9th position of each metal-binding site with asparagine, histidine, alanine, methionine, and selenomethionine. Spectroscopic measurements with lanthanides (Nd3+ and Eu3+) and actinides (243Am3+ and 248Cm3+) reveal that, contrary to the behavior of small chelator complexes, metal-coordinated water molecules enhance LanM’s affinity for f-elements and pH-stability of its complexes. Furthermore, the results show that the native aspartate does not coordinate the metal directly but rather hydrogen bonds to coordinated solvent. By tuning this first-sphere/second-sphere interaction, the asparagine variant nearly doubles LanM’s selectivity for actinides versus lanthanides. This study not only clarifies the essential role of coordinated solvent for LanM’s physiological function and separation applications, but it also demonstrates that LanM’s preference for actinides over lanthanides can be further improved. More broadly, it demonstrates how biomolecular scaffolds possess an expanded repertoire of tunable interactions compared to most small-molecule ligands – providing an avenue for high-performance LanM-based actinide/lanthanide separation methods and bio-engineered chelators optimized for specific medical isotopes.