2025-04-15 ノースウェスタン大学
<関連情報>
- https://news.northwestern.edu/stories/2025/04/uncovering-the-hidden-cost-of-water-splitting/
- https://www.nature.com/articles/s41467-025-58842-y
- https://www.science.org/doi/10.1126/sciadv.ado8536
Fe2O3ナノ層上での水の反転と酸素発生反応 Water flipping and the oxygen evolution reaction on Fe2O3 nanolayers
Raiden Speelman,Ezra J. Marker,Mavis D. Boamah,Jacob Kupferberg,Justin Z. Bye,Mark Engelhard,Yatong Zhao,Alex B. F. Martinson,Kevin M. Rosso & Franz M. Geiger
Nature Communications Published:15 April 2025
DOI:https://doi.org/10.1038/s41467-025-58842-y
Abstract
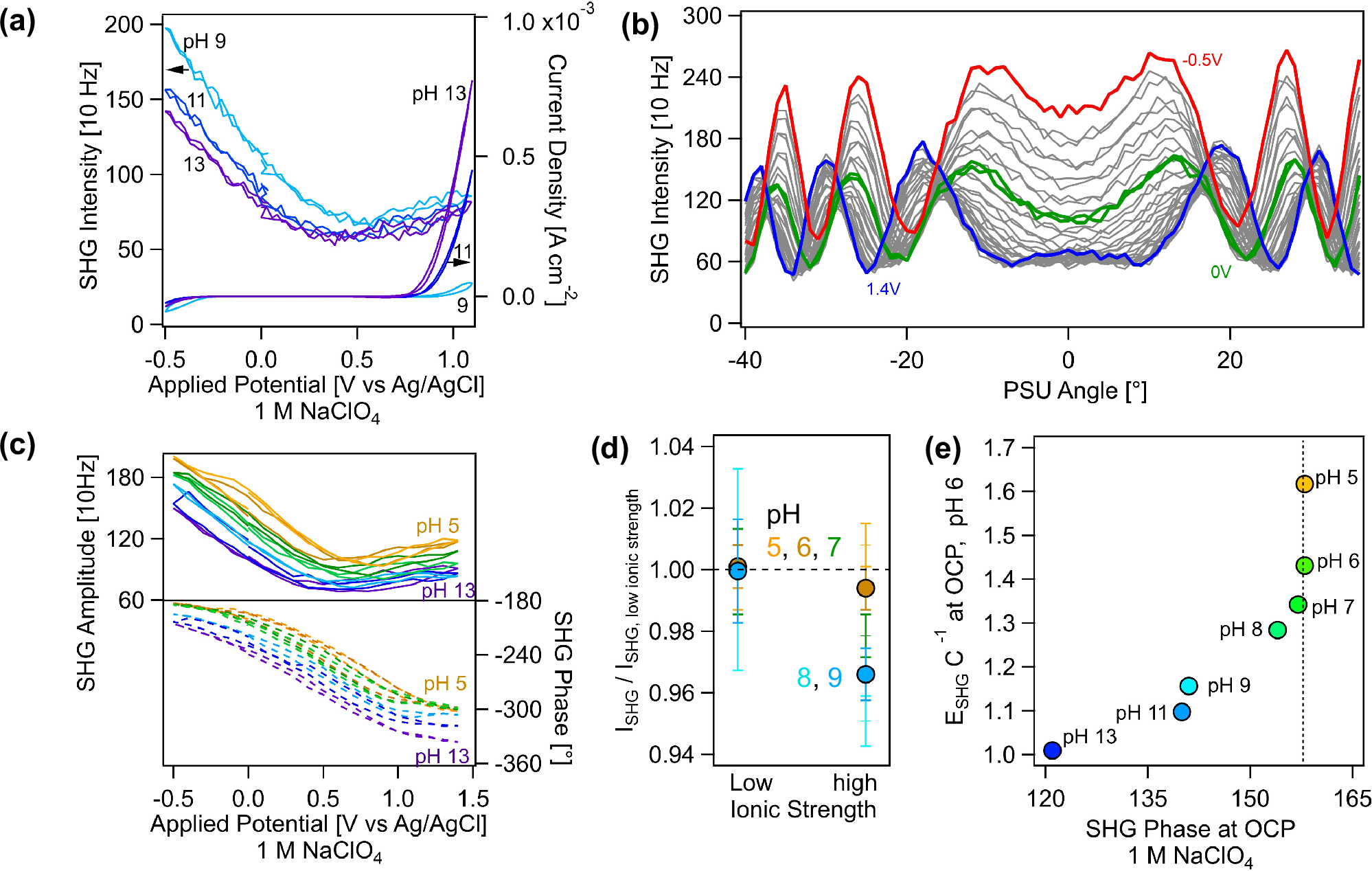
Hematite photoanodes are promising for the oxygen evolution reaction, however, their high overpotential (0.5-0.6 V) for water oxidation and limited photocurrent make them economically unviable at present. The work needed to orient dipoles at an electrode surface may be an overlooked contribution to the overpotential, especially regarding dipoles of water, the electron source in the oxygen evolution reaction (OER). Here, we employ second harmonic amplitude and phase measurements to quantify the number of net-aligned Stern layer water molecules and the work associated with water flipping, on hematite, an earth abundant OER semiconductor associated with a high overpotential. At zero applied bias, the pH-dependent potentials for Stern layer water molecule flipping exhibit Nernstian behavior. At positive applied potentials and pH 13, approximately one to two monolayers of water molecules points the oxygen atoms towards the electrode, favorable for the OER. The work associated with water flipping matches the cohesive energy of liquid water (44 kJ mol-1) and the OER current density is highest. This current is negligible at pH 5, where the work approaches 100 kJ mol-1. Our findings suggest a causal relationship between the need for Stern layer water flipping and the OER overpotential, which may lead to developing strategies for decreasing the latter.
酸素発生反応前および反応中のスターン層水アラインメントの定量化 Quantifying Stern layer water alignment before and during the oxygen evolution reaction
Raiden Speelman, Ezra J. Marker, and Franz M. Geiger
Science Advances Published:5 Mar 2025
DOI:https://doi.org/10.1126/sciadv.ado8536

Abstract
While water’s oxygen is the electron source in the industrially important oxygen evolution reaction, the strong absorber problem clouds our view of how the Stern layer water molecules orient themselves in response to applied potentials. Here, we report nonlinear optical measurements on nickel electrodes held at pH 13 indicating a disorder-to-order transition in the Stern layer water molecules before the onset of Faradaic current. A full water monolayer (1.1 × 1015 centimeter−2) aligns with oxygen atoms pointing toward the electrode at +0.8 volt and the associated work is 80 kilojoule per mole. Our experiments identify water flipping energetics as a target for understanding overpotentials, advance molecular electrochemistry, provide benchmarks for electrical double layer models, and serve as a diagnostic tool for understanding electrocatalysis.