2022-09-12 ノースカロライナ州立大学(NCState)
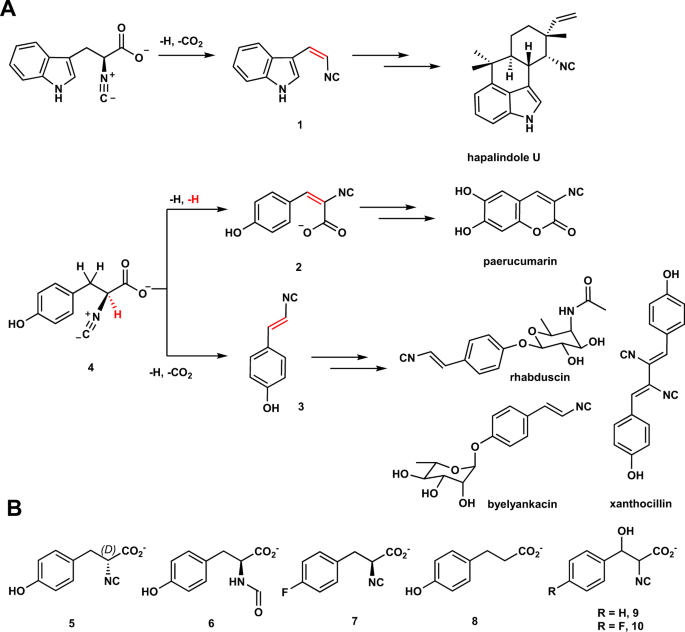
研究チームは、2種類のFe/2OG酵素(PvcBとPlsnB)に着目し、その構造と生成物を比較した。両酵素とも結合部位を特定したが、酵素がどのように変換を行うかを調べたところ、酵素が基質に結合し、分子状酸素(O2)の酸素原子が基質に導入され、酸素が付加されて反応が進行するという発見があった。
<関連情報>

ビニルイソニトリルおよびイソシアノアクリレートの生成における分岐した脱飽和経路の解明 Elucidation of divergent desaturation pathways in the formation of vinyl isonitrile and isocyanoacrylate
Wantae Kim,Tzu-Yu Chen,Lide Cha,Grace Zhou,Kristi Xing,Nicholas Koenig Canty,Yan Zhang & Wei-chen Chang
Nature Communications Published:12 September 2022
DOI:https://doi.org/10.1038/s41467-022-32870-4
Abstract
Two different types of desaturations are employed by iron- and 2-oxoglutarate-dependent (Fe/2OG) enzymes to construct vinyl isonitrile and isocyanoacrylate moieties found in isonitrile-containing natural products. A substrate-bound protein structure reveals a plausible strategy to affect desaturation and hints at substrate promiscuity of these enzymes. Analogs are synthesized and used as mechanistic probes to validate structural observations. Instead of proceeding through hydroxylated intermediate as previously proposed, a plausible carbocation species is utilized to trigger C=C bond installation. These Fe/2OG enzymes can also accommodate analogs with opposite chirality and different functional groups including isonitrile-(D)-tyrosine, N-formyl tyrosine, and phloretic acid, while maintaining the reaction selectivity.