安価な触媒で光エネルギーを利用し、アンモニアを水素燃料に変えることに成功 Inexpensive catalyst uses energy from light to turn ammonia into hydrogen fuel
2022-11-24 ライス大学
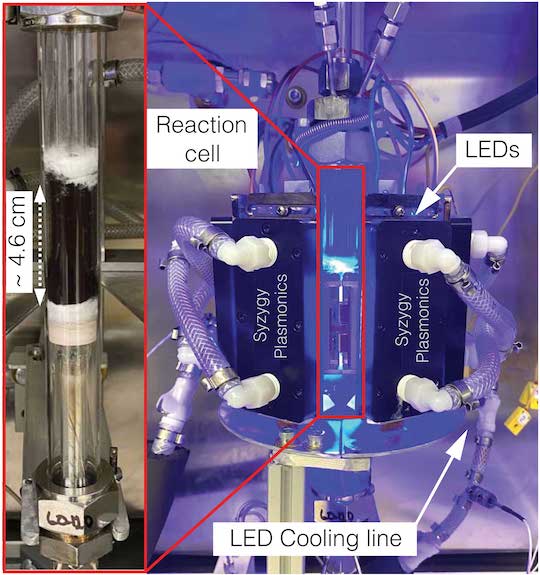
A reaction cell (left) and the photocatalytic platform (right) used on tests of copper-iron plasmonic photocatalysts for hydrogen production from ammonia at Syzygy Plasmonics in Houston. All reaction energy for the catalysis came from LEDs that produced light with a wavelength of 470 nanometers. Courtesy of Syzygy Plasmonics, Inc.
液体アンモニアは、1分子に窒素原子1個と水素原子3個を含むため、輸送が容易で、多くのエネルギーを含んでいる。新しい触媒は、この分子をクリーンな燃料である水素ガスと、地球大気の最大の構成要素である窒素ガスに分解する。また、従来の触媒とは異なり、熱を必要としない。その代わり、太陽光やエネルギー消費の少ないLEDなどの光からエネルギーを得ることができる。
この研究は、鉄が効率的なプラズモン光触媒になり得ることを示している。また、光触媒は、安価なLED光源で効率的に行えることも実証している。
研究者たちは、銅と鉄でできたアンテナリアクター粒子がアンモニアの変換に非常に有効であることを明らかにした。この粒子の銅製のエネルギーハーベスティング部分は、可視光からエネルギーを取り込む。
<関連情報>
- https://news.rice.edu/news/2022/rice-labs-catalyst-could-be-key-hydrogen-economy
- https://www.science.org/doi/10.1126/science.abn5636
発光ダイオード照射によるNH3からのH2生成のための地球資源の豊富な光触媒 Earth-abundant photocatalyst for H2 generation from NH3 with light-emitting diode illumination
Yigao Yuan,Linan Zhou,Hossein Robatjazi ,Junwei Lucas Bao,Jingyi Zhou,Aaron Bayles,Lin Yuan ,Minghe Lou,Minhan Lou,Suman Khatiwada,Emily A. Carter,Peter Nordlander,Naomi J. Halas
Science Published:24 Nov 2022
DOI: 10.1126/science.abn5636
Harnessing iron for photocatalysis
Good catalysts bind substrates at intermediate strengths so that neither reactant binding nor product desorption limits the reaction. Platinum group metals often meet this criterion for many reactions and cannot be replaced with cheaper metals such as iron, which often oxidize under reaction conditions. Yuan et al. demonstrate ammonia decomposition to liberate hydrogen with a copper-iron photocatalyst in which plasmons excited in copper generate hot electrons that react with ammonia bound to iron. Iron is not a good thermal catalyst for this reaction, but photoinduced oxygen desorption makes it competitive with a similar copper–ruthenium photocatalyst and with ruthenium thermal catalysts. This reaction, which is driven with light-emitting diodes, may be competitive with thermal catalysts used in this hydrogen carrier system. —PDS
Abstract
Catalysts based on platinum group metals have been a major focus of the chemical industry for decades. We show that plasmonic photocatalysis can transform a thermally unreactive, earth-abundant transition metal into a catalytically active site under illumination. Fe active sites in a Cu-Fe antenna-reactor complex achieve efficiencies very similar to Ru for the photocatalytic decomposition of ammonia under ultrafast pulsed illumination. When illuminated with light-emitting diodes rather than lasers, the photocatalytic efficiencies remain comparable, even when the scale of reaction increases by nearly three orders of magnitude. This result demonstrates the potential for highly efficient, electrically driven production of hydrogen from an ammonia carrier with earth-abundant transition metals.