2022-03-31 ミシガン工科大学
・PFASは、そのユニークな特性から、撥水加工された衣類や焦げ付きにくい調理器具、ピザの箱、スキーワックス、ファーストフードの包み紙、消火用泡など、日常生活のあらゆるところで使用されています。
・「PFASは非常に強い炭素-フッ素結合を持ち、生物活動によって容易に分解されません。PFASは、ほぼ永遠に環境中に存在し続けるため、”永遠の化学物質 ”と呼ばれています。地下水や地表水、水路、そして最終的には飲料水や生態系(淡水魚を含む)も汚染してしまう。」と、土木・環境・地理空間工学の南方大輔准教授は語る。
<関連情報>
- https://www.mtu.edu/news/2022/03/farewell-forever-chemicals-researchers-aim-to-eliminate-pfas-for-good.html
- https://pubs.rsc.org/en/content/articlelanding/2022/ew/d1ew00897h
水相の高度な還元プロセスにおける水和電子と有機化合物の反応性 Reactivities of hydrated electrons with organic compounds in aqueous-phase advanced reduction processes
Rose Daily and Daisuke Minakata Royal Society ofChemistry
Abstract
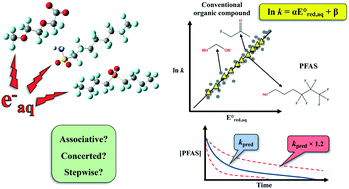
Advanced reduction processes (ARPs) that generate reactive electrons in homogeneous solution and heterogeneous electrochemical or catalytic processes are effective in degrading oxidized forms of organic and inorganic contaminants. However, the detailed mechanisms of compounds with multiple functional groups and the effect of those functional groups on the reactivities of these compounds toward electrons have not been elucidated. In this study, we use density functional theory to calculate the aqueous-phase one electron reduction potential 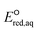 of 251 conventional organic compounds containing a wide variety of functional groups. We investigate three possible elementary reaction mechanisms, namely, the associative, concerted and stepwise cleavage mechanisms, at all possible reactive sites and determine the linear free energy relationships (LFERs) between the experimentally measured rate constants of hydrated electrons (eaq−) and the
of 251 conventional organic compounds containing a wide variety of functional groups. We investigate three possible elementary reaction mechanisms, namely, the associative, concerted and stepwise cleavage mechanisms, at all possible reactive sites and determine the linear free energy relationships (LFERs) between the experimentally measured rate constants of hydrated electrons (eaq−) and the 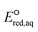 values. In addition, we use the 75 priority per- and polyfluoroalkyl substance (PFAS) subsets from the United States Environmental Protection Agency (U.S. EPA) to calculate the
values. In addition, we use the 75 priority per- and polyfluoroalkyl substance (PFAS) subsets from the United States Environmental Protection Agency (U.S. EPA) to calculate the 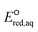 values of all possible elementary reactions of each PFAS to determine their dominant reaction mechanisms and reactive sites. LFERs of conventional organic compounds are used to predict the reactivities of eaq− with PFASs, which can be used as a screening tool to evaluate the electron-induced degradability of thousands of PFASs for both homogeneous and heterogeneous reduction processes. Finally, we develop a kinetic model to investigate the impact of an accurate rate constant prediction on the fate of an environmentally relevant organic compound induced by eaq− in a homogeneous aqueous-phase ARP.
values of all possible elementary reactions of each PFAS to determine their dominant reaction mechanisms and reactive sites. LFERs of conventional organic compounds are used to predict the reactivities of eaq− with PFASs, which can be used as a screening tool to evaluate the electron-induced degradability of thousands of PFASs for both homogeneous and heterogeneous reduction processes. Finally, we develop a kinetic model to investigate the impact of an accurate rate constant prediction on the fate of an environmentally relevant organic compound induced by eaq− in a homogeneous aqueous-phase ARP.