2026-01-09 東京科学大学
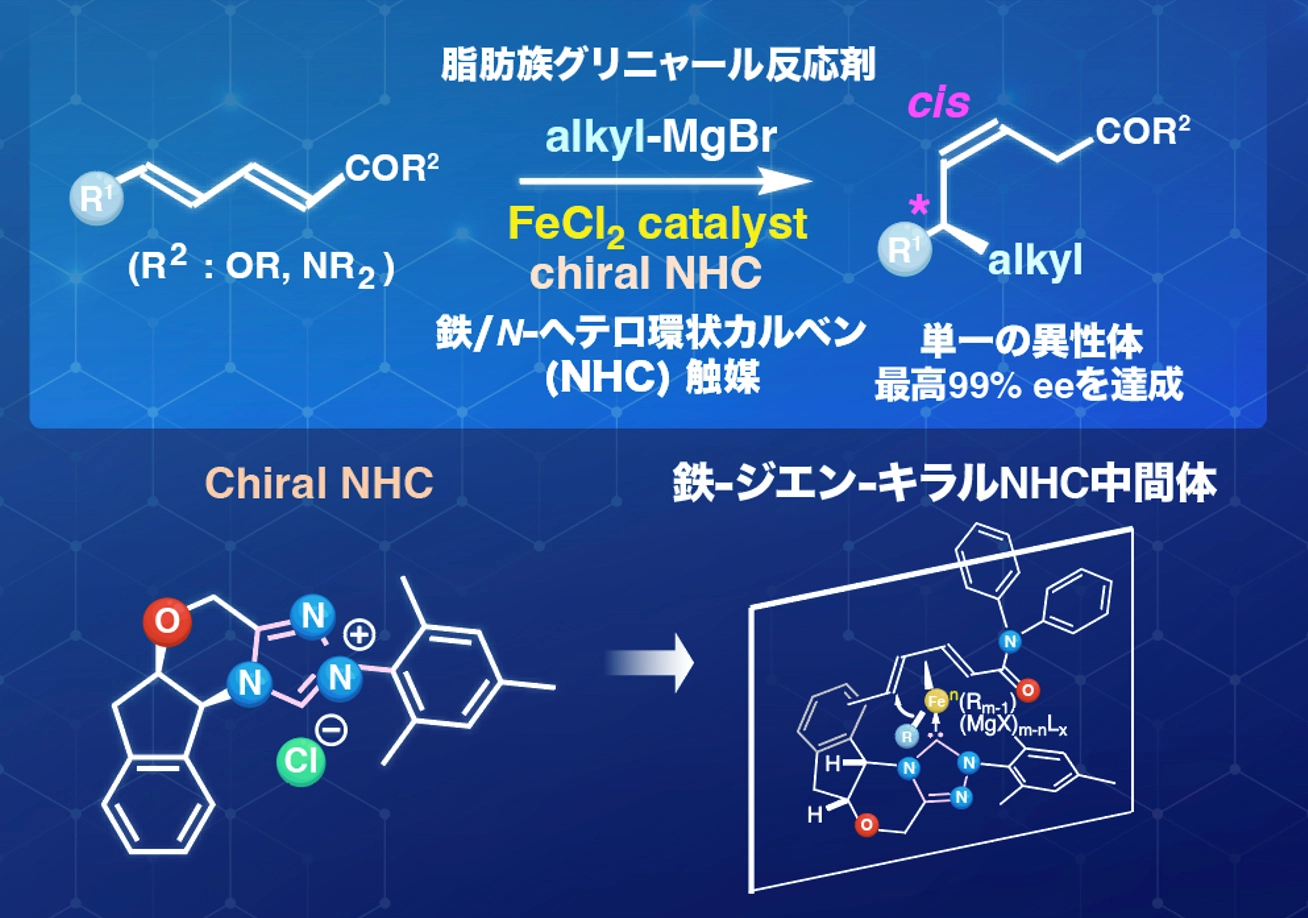
図. 鉄/キラルNHC触媒による脂肪族グリニャール反応剤の α,β,γ,δ-不飽和カルボニル化合物への不斉1,6-付加反応
<関連情報>
- https://www.isct.ac.jp/ja/news/5bqg53ywv2tv
- https://onlinelibrary.wiley.com/doi/10.1002/anie.202518346
鉄/NHC触媒を用いた脂肪族グリニャール試薬のα,β,γ,δ-不飽和カルボニル化合物への位置選択的および立体選択的1,6-付加反応:キラルNHCを用いた不斉変種 Iron/NHC-Catalyzed Regio- and Stereoselective 1,6-Additions of Aliphatic Grignard Reagents to α,β,γ,δ-Unsaturated Carbonyl Compounds: Asymmetric Variants with Chiral NHCs
Kazuma Abe, Koki Nishi, Hirokazu Ito, Shoma Kobayashi, Shumpei Saito, Prof. Dr. Takeshi Hata
Angewandte Chemie International Edition Published: 06 November 2025
DOI:https://doi.org/10.1002/anie.202518346
Abstract
Although conjugate addition to an α,β-unsaturated carbonyl compound is a well-established method in synthetic organic chemistry, controlling regioselectivity, olefin geometry, and positional isomerism in analogous 1,6-addition chemistry, which provides a powerful approach for constructing molecular complexity, remains a key challenge. Herein, we report the iron-catalyzed regio-, stereo-, and enantioselective 1,6-additions of aliphatic Grignard reagents to α,β,γ,δ-unsaturated carbonyl compounds. The incorporation of an N-heterocyclic carbene (NHC) ligand effectively suppresses undesired β-hydride elimination, thereby enabling the highly cis-selective installation of the aliphatic group. Remarkably, the use of a rigid tetracyclic chiral NHC ligand afforded the corresponding adducts with enantioselectivities of up to 99%. The developed reaction shows broad substrate scope, accommodating various aliphatic Grignard reagents and unsaturated carbonyl compounds, thereby providing direct access to optically active 1,6-adducts bearing cis-configured olefins. Mechanistic investigations, including deuterium-labeling experiments, support the involvement of magnesium enolates and an iron–NHC catalytic cycle. The developed transformation provides a powerful strategy for the remote functionalization of extended conjugated carbonyl frameworks.