2025-09-04 東京科学大学
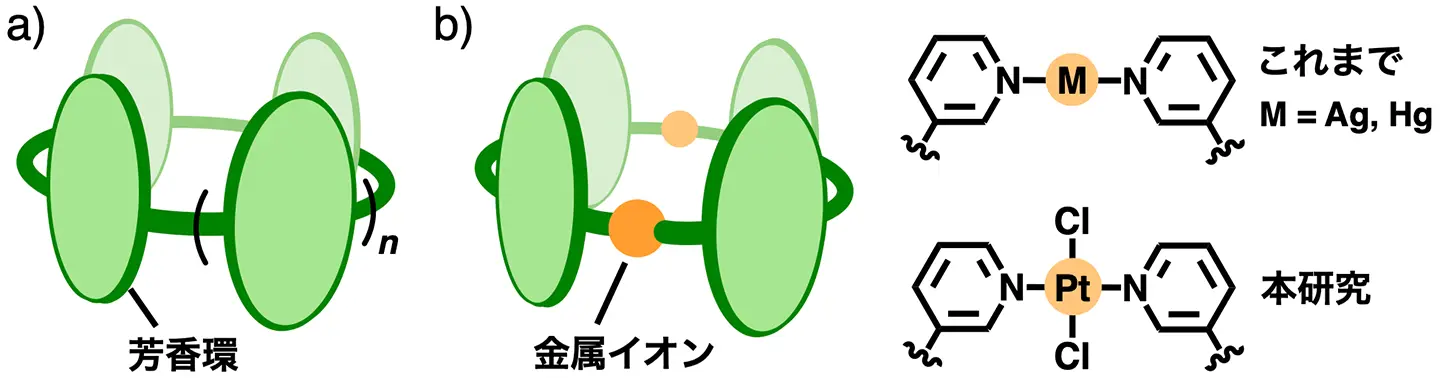
図1. a)これまでの芳香環リング・チューブ,b)配位結合で作製した芳香環チューブ:これまで(上)と本研究(下)の分子設計。
<関連情報>
- https://www.isct.ac.jp/ja/news/aay4pr7luuc9
- https://onlinelibrary.wiley.com/doi/10.1002/anie.202510929
二重多機能化能力を有する多芳香族金属チューブ A Polyaromatic Metallotube With a Dual Multi-Functionalization Ability
Koki Tagai, Dr. Yuya Tanaka, Prof. Dr. Michio Yamada, Prof. Dr. Michito Yoshizawa
Angewandte Chemie International Edition Published: 17 June 2025
DOI:https://doi.org/10.1002/anie.202510929
Graphical Abstract
A new polyaromatic metallotube, formed from PtCl2 and bispyridine ligands, undergoes dual multi-functionalization via substitution on the metal hinges. The first multi-functionalized Pt(II)-tube exhibits high water solubility as well as binds one molecule of fullerene C60, which is subsequently released through the second multi-functionalization, in a quantitative fashion.

Abstract
Post-functionalization is an advanced, key synthetic method in supramolecular systems, whereas the previous method relies heavily on the substitution on the organic frameworks. Here we report a polyaromatic metallotube capable of dually multi-functionalizing the metal sites in an efficient post-assembly fashion. The Pt(II)-linked tube with tetrachloro groups is prepared from two PtCl2 hinges and two bent bispyridine ligands in high yield. The new metallotube efficiently converts to tetra-substituted tubes by the first multiple functionalization, through the replacement of the chloro groups with various alkynyl groups. The tubular structures before/after the functionalization are unequivocally confirmed by X-ray crystallographic analysis. A resultant tetraammonium-attached Pt(II)-tube exhibits high water solubility and uptake abilities toward spherical and rod-like compounds. The metallotube strongly binds one molecule of fullerene C60 in the polyaromatic cavity yet releases it through the second multi-functionalization by tetrabromination of the metal hinges in a quantitative and reversible manner. Even after Bingel reaction within the tube, sterically demanding bis-substituted fullerenes (>∼80% selectivity) are readily released by the second functionalization. The present method, i.e., the use of simple PtCl2 hinges, will be applicable to wide-ranging metallo-supramolecules to enhance their structural and functional versatilities via post-multi-functionalizations.