2024-04-23 パシフィック・ノースウェスト国立研究所(PNNL)
<関連情報>
- https://www.pnnl.gov/news-media/critical-minerals-recovery-electronic-waste
- https://pubs.rsc.org/en/content/articlelanding/2024/su/d3su00403a#cit2
- https://pubs.acs.org/doi/full/10.1021/acs.estlett.3c00754
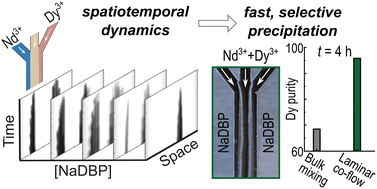
水溶液からのネオジムおよびジスプロシウム分離の流れによる促進 Flow-driven enhancement of neodymium and dysprosium separation from aqueous solutions
Qingpu Wang and Chinmayee V. Subban
RSC Sustainability Published:12 Feb 2024
DOI:https://doi.org/10.1039/D3SU00403A
Abstract
Selective extraction of rare earth elements (REEs) from waste NdFeB magnets and natural mineral sources has been challenging due to the similar properties of neodymium (Nd) and dysprosium (Dy). Current separation methods mainly include solvent extraction and organic ligand-based selective precipitation, which are chemical- and energy-intensive in addition to the long separation times required to reach thermodynamic equilibrium. Here, we demonstrate a laminar co-flow method that relies on flow-induced non-equilibrium conditions to selectively precipitate Dy3+ from aqueous solutions containing mixed Nd3+ and Dy3+ at various ratios. The concentration of reactant sodium dibutyl phosphate for selective precipitation was identified based on the differences in the spatiotemporal dynamics of the Nd3+ and Dy3+ precipitates. Under optimized conditions, our method showed increased Dy purity in the precipitate product at significantly shorter reaction times, compared to commonly used convective bulk mixing. We found a nearly Dy-pure (99.9%) precipitate from starting mixtures of Nd : Dy in 50 : 50 and 30 : 70 ratios. Our single-step method is efficient and environmentally friendly and does not require harmful organic solvents or difficult to synthesize complex ligands.
反応拡散カップリングが電池原料水溶液からの金属イオンの逐次析出を促進する Reaction–Diffusion Coupling Facilitates the Sequential Precipitation of Metal Ions from Battery Feedstock Solutions
Qingpu Wang and Elias Nakouzi
Environmental Science & Technology Letters Published:November 22, 2023
DOI:https://doi.org/10.1021/acs.estlett.3c00754
Abstract

The development of new technologies for chemical separations is urgently needed to meet the surging demand for critical materials that has strained resources and caused environmental challenges. Inspired by the classic Liesegang experiment, we demonstrated the separation of critical metal ions based on the coupling of ion diffusion and precipitation kinetics. For this purpose, a model feedstock solution simulating dissolved battery electrodes was placed on top of a hydrogel loaded with a precipitating agent, namely, sodium hydroxide. As the lithium, manganese, cobalt, and nickel ions diffused into the gel, a gradient of precipitates formed along the length of the reactor. Elemental analysis of the spatially distributed precipitates showed the enrichment of nickel near the gel–solution interface, followed by the formation of an almost pure (>96%) manganese product further along the reactor. Optimization experiments revealed that a sodium hydroxide concentration of 10 mM and a gel/solution volume ratio of 2:1 favored efficient separations. The robustness of the method was demonstrated in four out of five feedstock compositions of typically used battery cathodes. Our proof-of-concept experiments present a paradigm for critical materials separations that does not require specialty chemicals, binding agents, membranes, or toxic solvents.