2024-04-26 パシフィック・ノースウェスト国立研究所(PNNL)
<関連情報>
- https://www.pnnl.gov/publications/heavy-metal-complexation-ionic-liquids-enables-efficient-electrochemical-separation
- https://pubs.rsc.org/en/content/articlehtml/2024/gc/d3gc03713d
重金属カチオンとイミダゾリウムイオン液体の錯形成による還元エネルギーの低下:電気化学的分離への影響 Complexation of heavy metal cations with imidazolium ionic liquids lowers their reduction energy: implications for electrochemical separations
Shuai Tan, Difan Zhang , Ying Chen, Benjamin A. Helfrecht, Eric T. Baxter, Wenjin Cao, Xue-Bin Wang, Manh-Thuong Nguyen, Grant E. Johnson and Venkateshkumar Prabhakaran
Green Chemistry Published on 22nd December 2023
DOI:https://doi.org/10.1039/D3GC03713D
Abstract
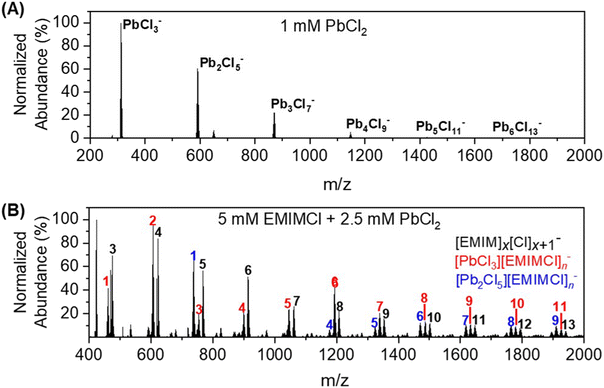
Ionic liquids (ILs) are emerging as promising materials for the separation of heavy metals from complex feed streams through selective complexation. A predictive understanding of the coordination chemistry between ILs and targeted metal ions is critically important for enabling the rational design of efficient and selective separations. Such understanding is challenging to obtain due to the labile nature of the bonds and complicated structures formed by ILs in solution. Herein, we elucidated the complex formation of imidazolium-based ILs (i.e., 1-ethyl-3-methylimidazolium chloride, EMIMCl) with lead cations (Pb2+) in both the gas and aqueous phases employing a combination of experimental and theoretical methods. Gas-phase electrospray ionization mass spectrometry (ESI-MS) and negative ion photoelectron spectroscopy (NIPES) experiments suggest that Pb–Cl anions (e.g., PbCl3− and Pb2Cl5−) combine with neutral EMIMCl molecules, forming complexes of [PbmCl2m+1][EMIMCl]n− (m = 1, 2, n = 1–4). These anionic complexes are shown to become less stable toward fragmentation and require more energy to dissociate an electron with an increasing number of EMIMCl molecules. In contrast, in dilute solutions, dissociated EMIM+ cations and Cl− anions give rise to electrostatic screening of Pb–Cl bonds, resulting in the formation of distinct condensed phase complexes, such as [PbCl5][EMIM]2. These structures were identified by nuclear magnetic resonance (NMR) spectroscopy coupled with density functional theory (DFT) calculations. The energy gap between the highest occupied and lowest unoccupied molecular orbitals (HOMO–LUMO) of the condensed phase complexes containing EMIM+ was calculated to be lower compared to the Pb–Cl ionic clusters without IL, making these species more electrochemically reducible and easier to extract from solution. This study emphasizes the importance of understanding complexation between ILs and metal ions in designing efficient separation methods.