2026-01-30 早稲田大学
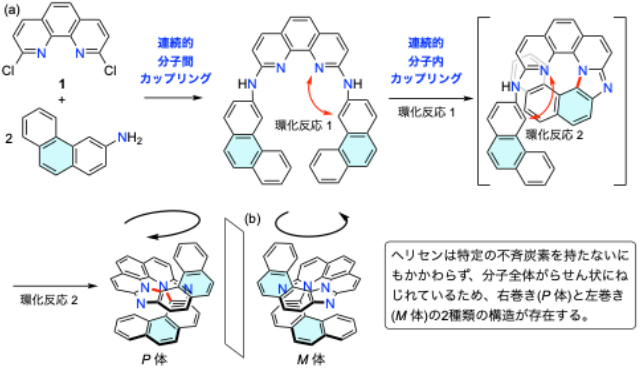
図1.(a) テトラアザ[11]ヘリセンを例とした本研究で開発した2段階合成法と(b)ヘリセンの鏡像異性体の構造
<関連情報>
- https://www.waseda.jp/inst/research/news/83386
- https://onlinelibrary.wiley.com/doi/10.1002/anie.202524463
二段階戦略によるテトラアザ[7]-[15]ヘリセンの合成:増幅円偏光発光を示す長さ制御キラルπ-システム Tetraaza[7]–[15]helicenes Synthesized by Two-Step Strategy: Length-Controlled Chiral π-Systems Exhibiting Amplified Circularly Polarized Luminescence
Prof. Dr. Takashi Otani, Yuchen Wu, Prof. Dr. Kohei Ueda, Yuki Ikeda, Yuna Tada, Natsuna Kinoshita, Prof. Dr. Takanori Shibata
Angewandte Chemie International Edition Published:published: 09 January 2026
DOI:https://doi.org/10.1002/anie.202524463
Abstract
Helicenes are chiral π-conjugated molecules whose properties strongly depend on their lengths. Systematic studies of these compounds have been limited by synthetic challenges. Here we report a concise two-step strategy (defined as the helicene-forming sequence from aminohelicene precursors) to access a homologous series of tetraaza[7]–[15]helicenes. Optical spectra converge beyond [11]H, defining a conjugation ceiling, while chiroptical responses amplify sharply, yielding |glum| up to 0.028. Fluorescence quantum yields show a nonmonotonic dependence, with [7]H and [15]H maintaining both high ΦF (0.39 and 0.36) and large |glum|, resulting in outstanding, albeit semi-quantitative, CPL performance, with figures of merit reaching 0.010 and CPL brightness values of approximately 490. TD-DFT calculations attribute this amplification to the delayed alignment of electric and magnetic transition dipoles, while 1H NMR shifts of inner protons provide an independent probe of structural reorganization within the helical cavity. Additionally, experiment and theory have consistently identified [11]H as the critical helicene length at which the framework undergoes a qualitative transition. Notably, the [15]helicene constitutes the longest helicene ever resolved into its enantiomers, underscoring the synthetic power of this modular approach. Importantly, our synthetic route is effective for constructing higher-order helicenes, offering a generalizable platform for length-controlled, heteroatom-containing helicenes. These findings establish long tetraazahelicenes as a rare platform where through-bond conjugation and through-space orbital coupling act cooperatively to govern photophysical and chiroptical properties.