2025-10-10 早稲田大学
<関連情報>
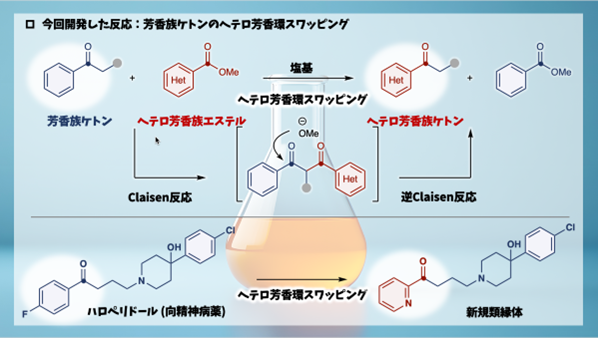
芳香族ケトンにおけるヘテロ芳香族交換 Heteroaromatic swapping in aromatic ketones
Hikaru Nakahara,Ryotaro Shirai,Yoshio Nishimoto,Daisuke Yokogawa & Junichiro Yamaguchi
Nature Communications Published:09 October 2025
DOI:https://doi.org/10.1038/s41467-025-64041-6
Abstract
The modification of aromatic rings to heteroaromatic rings is a widely employed strategy in medicinal chemistry, often used to modulate lipophilicity and improve metabolic stability. However, achieving a one-step, generalizable transformation of aromatic rings into diverse heteroaromatic rings—termed “heteroaromatic swapping”—remains a persistent challenge. Existing methods, such as skeletal editing and transition-metal-catalyzed aromatic ring exchange, are limited in substrate scope and efficiency. Here, we present an efficient strategy for heteroaromatic swapping via a Claisen/retro-Claisen mechanism, utilizing heteroaryl esters and aromatic ketones. This approach enables the selective exchange of aromatic rings with heteroaromatic rings across a broad substrate range, overcoming the limitations of existing techniques. Notably, it achieves high-yield conversions of bioactive aromatic ketones into their heteroaromatic counterparts. This method expands the molecular editing toolkit, offering a practical and versatile platform for synthesizing bioactive compounds with enhanced physicochemical properties.