2025-07-21 早稲田大学
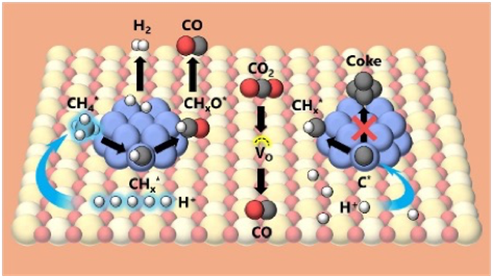
図 左が今回発見したスムーズに進む方法、右が炭素(coke)で汚れやすい従来の方法
<関連情報>
高圧下での炭素析出を抑制する電気的アシストによるメタンの低温乾式改質 Electrically Assisted Low-Temperature Dry Reforming of Methane Suppressing Carbon Deposition under High-Pressure Conditions
Clarence Sampson,Takumi Masuda,Taisuke Horiguchi,Saori Ichiguchi,Hiroshi Sampei,Hitoshi Matsubara,Shintaro Itagaki,Gen Inoue,and Yasushi Sekine
ACS Catalysis Published: July 18, 2025
DOI:https://doi.org/10.1021/acscatal.5c03126
Abstract
Our approach to high-pressure dry reforming of methane (DRM) achieves synergistic performance enhancement via application of an electric field (EF) over a 1 wt % Ru/La2Ce2O7 (LCO) catalyst. Conventional DRM is adversely affected by compromised activity and catalyst stability under pressurization caused by unfavorable thermodynamics. In sharp contrast, EF-assisted DRM has achieved exceptional CH4/CO2 conversion. In fact, high H2/CO ratios show coke resistance at temperatures as low as 473 K, which exceeds the equilibrium conversion constraints observed for conventional DRM. Pressurization was found to further enhance EF-assisted DRM activity by increasing the surface coverage of adsorbates that facilitates surface protonics, which is a proton hopping mechanism that promotes CH4 dissociative adsorption at low temperatures. Raman measurements and TEM-EDX mapping results show remarkable suppression of carbon deposition and metal sintering as the cause of long-term durability of EF-assisted DRM. When elucidating the reaction mechanism, temperature dependence, and turnover frequency (TOF) investigations have indicated unconventional anti-Arrhenius behavior, particularly identifying the metal–support interface as the primary active site. Partial pressure-based kinetic studies and transient gas-switch test results suggest that CHxO species serve as key reaction intermediates capable of direct decomposition into H2 and CO. NNP-based structural optimization calculations identified CHO* as the most stable intermediate species formed through lattice oxygen interactions with CH4 dissociation. From C–H*, although oxidation into CHO* is kinetically favorable, dehydrogenation into C* is thermodynamically favorable. For rationalizing the distinct coke suppression shown by EF-assisted DRM, investigations of C–C* aggregation have revealed that C–H* formation grew increasingly more favorable over C–C* in the presence of high surface H concentrations. Granted that high H surface coverage was found in pressurized EF-assisted DRM, this growth indicates a potential H feedback mechanism that facilitates hydrogenation to C–H*, thereby suppressing coke formation.


