2025-07-11 京都大学
<関連情報>
- https://www.kyoto-u.ac.jp/ja/research-news/2025-07-11
- https://www.kyoto-u.ac.jp/sites/default/files/2025-07/web_2507_Mizuhata-e5a263934344c88d019a600d2349fb32.pdf
- https://onlinelibrary.wiley.com/doi/10.1002/anie.202508927
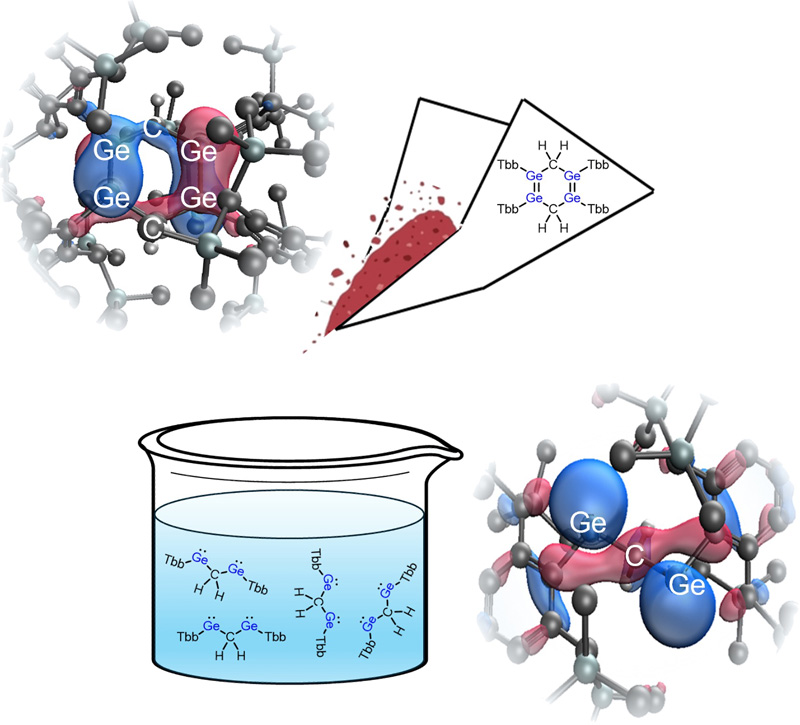
メチレン架橋1,3-ビス(ゲルミレン)の二量体との動的平衡における反応性 Reactivity of a Methylene-Bridged 1,3-Bis(germylene) in Dynamic Equilibrium with Its Dimer
Daichi Uchida, Prof. Dr. Mariko Yukimoto, Prof. Dr. Norihiro Tokitoh, Prof. Dr. Mitsuaki Yamauchi, Dr. Hiroko Yamada, Prof. Dr. Yoshiyuki Mizuhata
Angewandte Chemie International Edition Published: 23 June 2025
DOI:https://doi.org/10.1002/anie.202508927
Abstract
We report the properties and reactivity of an unprecedented methylene-bridged 1,3-bis(germylene) derivative. In the solid state, it undergoes dimerization to afford a 1,2,4,5-tetragermacyclohexa-1,4-diene derivative (Ge4CHD). Moreover, theoretical calculations on Ge4CHD reveal σ*–π interactions between the two π orbitals (Ge═Ge) and the CH σ* orbital. This interaction is confirmed by a significant red shift in the solid-state UV–vis spectrum. In contrast to the behavior in the solid state, the Ge4CHD derivative dissociates in solution into a methylene-bridged 1,3-bis(germylene) derivative. The resultant 1,3-bis(germylene) derivative reacted with S8 to form a novel cage compound containing three S and two Ge atoms. On the other hand, in the reaction with triphenylphosphine sulfide, the in situ generated 2-thia-1,3-digermabicyclo[1.1.0]butane derivative activated the benzene solvent, leading to the formation of a [2 + 2] cycloaddition product. Additionally, the 1,3-bis(germylene) derivative reacted with 4-dimethylaminopyridine (DMAP) to form a three-membered ring. Its structural parameters and the results of theoretical calculations indicated the zwitterionic character.



