2025-04-30 熊本大学
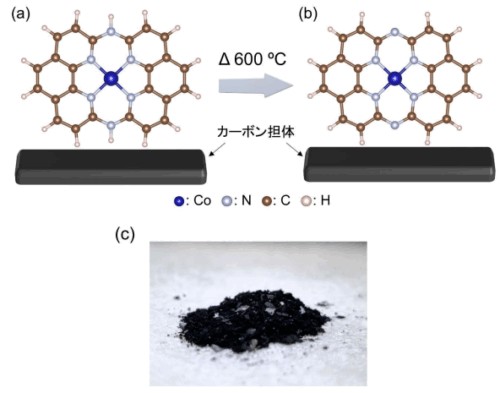
図 1. 本研究で開発した Co-14MR/C 触媒の模式図:(a)熱処理前、(b) 600℃ 熱処理後。(c)触媒粉末の写真。
<関連情報>
- https://www.kumamoto-u.ac.jp/whatsnew/sizen/20250430
- https://www.kumamoto-u.ac.jp/daigakujouhou/kouhou/pressrelease/2025_file/release250430.pdf
- https://pubs.acs.org/doi/10.1021/jacs.5c01306
酸素還元および水素発生反応のための耐久性があり活性な非白金族金属触媒の基礎となりうる14員環大環状コバルト錯体構造 Fourteen-Membered Macrocyclic Cobalt Complex Structure as a Potential Basis for Durable and Active Non-platinum Group Metal Catalysts for Oxygen Reduction and Hydrogen Evolution Reactions
Zhiqing Feng,Junya Ohyama,Soutaro Honda,Yasushi Iwata,Keisuke Awaya,Masato Machida,Masayuki Tsushida,Ryota Goto,Takeo Ichihara,Makoto Moriya,and Yuta Nabae
Journal of the American Chemical Society Published: April 25, 2025
DOI:https://doi.org/10.1021/jacs.5c01306
Abstract
Non-platinum group metal catalysts for the oxygen reduction reaction (ORR) and hydrogen evolution reaction (HER) under acidic conditions were developed using a CoN4 complex with a 14-membered-ring hexaazamacrocyclic ligand (Co-14MR). The carbon-supported Co-14MR catalyst (Co-14MR/C) showed higher ORR and HER activities than a conventional carbon-supported 16-membered-ring Co phthalocyanine (CoPc/C) catalyst. Heat treatment of Co-14MR/C at 600 °C further enhanced its ORR and HER activity through structural modification of the Co active center via deprotonation of ligand amine groups. Density functional theory calculations indicated that the structural modifications of Co-14MR induced by heat treatment adjusted the adsorption energies of important intermediates in the ORR and HER toward optimal values, resulting in enhanced catalytic activity. The Co-14MR/C catalysts also exhibited higher durability in the ORR and HER than CoPc/C and Fe-14MR/C catalysts. Structural analysis suggested that the short Co–N bond lengths and small distortion of the CoN4 active site of the Co-14MR catalysts are the reasons for their high durability. These findings suggest that the Co-14MR structure is a promising design for non-platinum group metal catalysts for proton-exchange membrane fuel cells and water splitting.