2024-05-29 パシフィック・ノースウェスト国立研究所(PNNL)
<関連情報>
- https://www.pnnl.gov/publications/establishing-novel-carbon-dioxide-chemistry-through-nano-clustering-water-lean
- https://www.nature.com/articles/s41557-024-01495-z
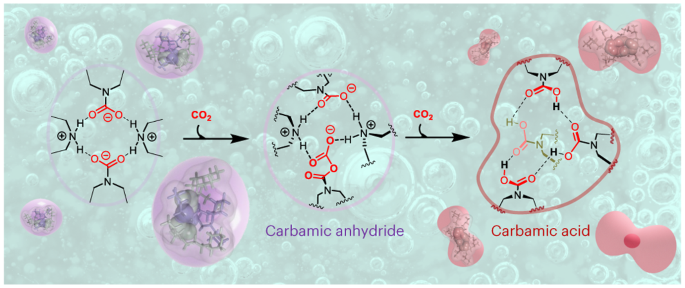
水溶性溶媒の四量体自己組織化により、カルバミン酸無水物ベースのCO2回収化学が可能になる Tetrameric self-assembling of water-lean solvents enables carbamate anhydride-based CO2 capture chemistry
Julien Leclaire,David J. Heldebrant,Katarzyna Grubel,Jean Septavaux,Marc Hennebelle,Eric Walter,Ying Chen,Jose Leobardo Bañuelos,Difan Zhang,Manh-Thuong Nguyen,Debmalya Ray,Sarah I. Allec,Deepika Malhotra,Wontae Joo & Jaelynne King
Abstract
Carbon capture, utilization and storage is a key yet cost-intensive technology for the fight against climate change. Single-component water-lean solvents have emerged as promising materials for post-combustion CO2 capture, but little is known regarding their mechanism of action. Here we present a combined experimental and modelling study of single-component water-lean solvents, and we find that CO2 capture is accompanied by the self-assembly of reverse-micelle-like tetrameric clusters in solution. This spontaneous aggregation leads to stepwise cooperative capture phenomena with highly contrasting mechanistic and thermodynamic features. The emergence of well-defined supramolecular architectures displaying a hydrogen-bonded internal core, reminiscent of enzymatic active sites, enables the formation of CO2-containing molecular species such as carbamic acid, carbamic anhydride and alkoxy carbamic anhydrides. This system extends the scope of adducts and mechanisms observed during carbon capture. It opens the way to materials with a higher CO2 storage capacity and provides a means for carbamates to potentially act as initiators for future oligomerization or polymerization of CO2.