2024-05-01 ミネソタ大学
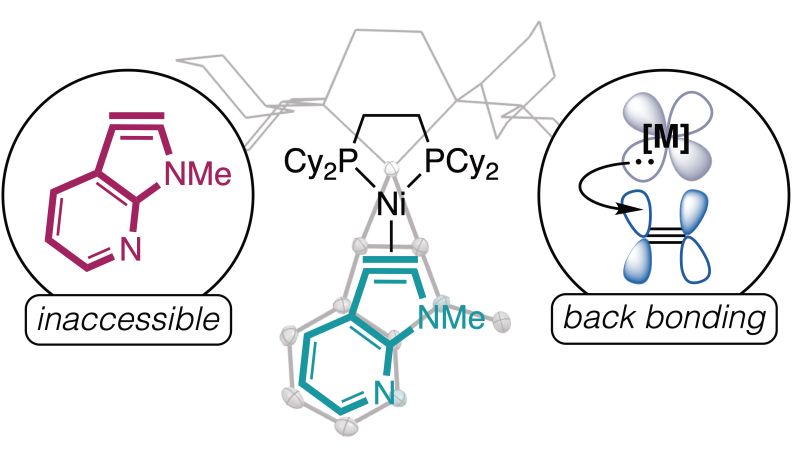
This graphic depicts the chemical compound that the team of chemists was able to discover. Credit: University of Minnesota
<関連情報>
- https://cse.umn.edu/college/news/researchers-create-new-chemical-compound-solve-120-year-old-problem
- https://www.science.org/doi/10.1126/science.adi1606
ニッケル結合により、これまでアクセスできなかった7-アザ-2,3-インドリンの単離と反応性が可能になった Nickel binding enables isolation and reactivity of previously inaccessible 7-aza-2,3-indolynes
JENNA N. HUMKE, ROMAN G. BELLI, ERIN E. PLASEK, SALLU S. KARGBO, […], AND COURTNEY C. ROBERTS
Science Published:25 Apr 2024
DOI:https://doi.org/10.1126/science.adi1606
Editor’s summary
How tightly can a carbon-carbon triple bond be confined? With the right driving reactions, it has become straightforward to squeeze the motif into six-membered rings such as benzyne and then reap the benefit of the prompt reactivity promoted by the strain. However, five-membered rings have tended to be too tight a squeeze. Humke et al. now report that coordination by nickel can relieve the strain just enough to stabilize a triple bond in the pentagonal portion of azaindoles. These azaindolyne complexes were crystallographically characterized and reacted with both nucleophiles and electrophiles. —Jake S. Yeston
Abstract
N-Heteroaromatics are key elements of pharmaceuticals, agrochemicals, and materials. N-Heteroarynes provide a scaffold to build these essential molecules but are underused because five-membered N-heteroarynes have been largely inaccessible on account of the strain of a triple bond in that small of a ring. On the basis of principles of metal-ligand interactions that are foundational to organometallic chemistry, in this work we report the stabilization of five-membered N-heteroarynes in the nickel coordination sphere. A series of 1,2-bis(dicyclohexylphosphino)ethane nickel 7-azaindol-2,3-yne complexes were synthesized and characterized crystallographically and spectroscopically. Ambiphilic reactivity of the nickel 7-azaindol-2,3-yne complexes was observed with multiple nucleophilic, electrophilic, and enophilic coupling partners.