2023-12-06 オークリッジ国立研究所(ORNL)
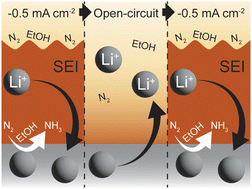
◆電気化学的プロセスを用い、窒素からアンモニアを生成する際の電流サイクルを中性子散乱で調査し、アンモニアの生成を増加させる手法を確立。これにより、農家は大気中の豊富な窒素を利用し、二酸化炭素排出を抑制した環境に優しい肥料を生産できる可能性があり、これが2050年までの世界的な炭素中立目標に貢献する可能性があります。
<関連情報>
- https://www.ornl.gov/news/neutrons-score-electrochemical-win-carbon-neutral-ammonia
- https://pubs.rsc.org/en/content/articlelanding/2023/ee/d2ee03694k
電気化学的N2還元過程における動的SEI形成の時間分解その場中性子反射率法およびX線回折法による複合解析 Combined, time-resolved, in situ neutron reflectometry and X-ray diffraction analysis of dynamic SEI formation during electrochemical N2 reduction
Sarah J. Blair, Mathieu Doucet, Valerie A. Niemann, Kevin H. Stone, Melissa E. Kreider, James F. Browning, Candice E. Halbert, Hanyu Wang, Peter Benedek, Eric J. McShane, Adam C. Nielander, Alessandro Gallo and Thomas F. Jaramillo
Energy & Environmental Science Published:28 Jun 2023
DOI:https://doi.org/10.1039/D2EE03694K
Abstract
One means of improving performance for electrochemical ammonia production through the Li-mediated N2 reduction reaction (Li-NRR) is by cycling the current driving the reaction between open-circuit conditions and periods of applied current density. Herein, we have investigated the dynamics of the electrode–electrolyte interface under Li-NRR conditions during current cycling using in situ time-resolved neutron reflectometry and grazing-incidence synchrotron X-ray diffraction. During cycling, measured neutron reflectivity curves indicated bilayer formation in which Li-containing species such as LiOH, Li2O, and small quantities of Li3N and metallic Li primarily appeared in a thin layer at the cathode surface, above which formed a much larger, porous, ‘solid–electrolyte interface’ (SEI) layer. Upon return to open-circuit conditions, Li-containing species quickly moved out of the thin layer, leaving a compact, stable layer of decomposition products underneath the SEI layer. This SEI layer concomitantly filled with electrolyte or dissolved, becoming indistinguishable from the electrolyte via contrast in scattering-length density (SLD). During the second current cycle, Li-containing species again preferentially deposited directly atop the cathode, with the thick SEI-like layer again appearing within a minute. This SEI layer exhibited a lower SLD more quickly than in the first cycle, which might suggest that Li-containing species become distributed within the porous SEI layer. Thus, these time-resolved observations of SEI and plated layers during current cycling suggest that benefits associated with return to open-circuit conditions between periods of applied current density may be related to the concomitant loss of Li-containing species from a thin layer at the cathode surface into a porous SEI layer that becomes filled with electrolyte or dissolves.