2025-10-06 マサチューセッツ工科大学(MIT)
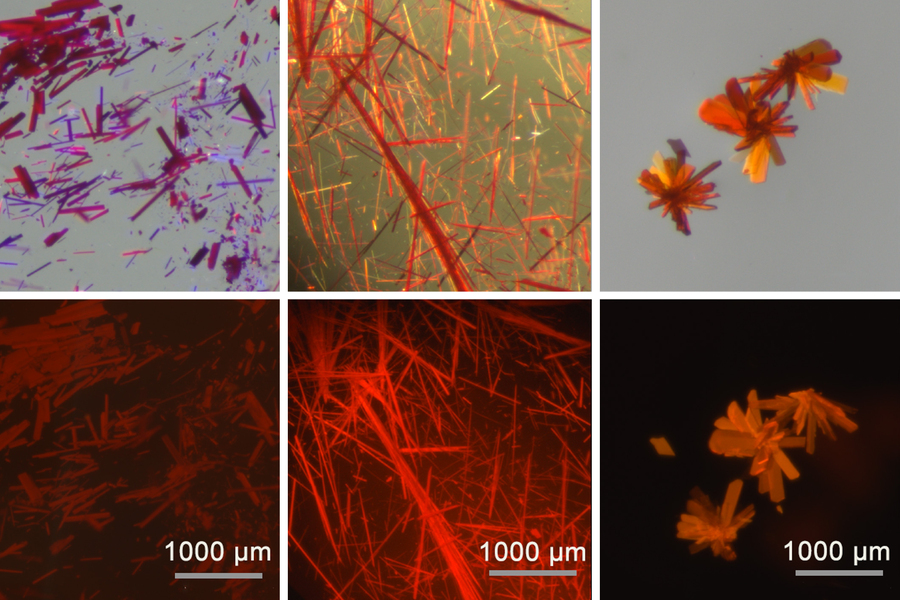
Caption:
MIT chemists have created a fluorescent, boron-containing molecule that is stable when exposed to air and can emit light in the red and near-infrared range. The dye can be made into crystals (shown in these images), films, or powders. The images at top were taken in ambient light and the images at bottom in UV light.
Credits:
Image: Courtesy of the researchers
<関連情報>
- https://news.mit.edu/2025/chemists-create-red-fluorescent-dyes-may-enable-clearer-biomedical-imaging-1006
- https://www.nature.com/articles/s41557-025-01941-6
- https://pubs.acs.org/doi/full/10.1021/jacs.1c11861
カルボジカルベンボレニウムイオンのイオン対形成による赤色から近赤外域の発光の解明 Unlocking red-to-near-infrared luminescence via ion-pair assembly in carbodicarbene borenium ions
Chun-Lin Deng,Bi Youan E. Tra,Xibao Zhang,Chonghe Zhang & Robert J. Gilliard Jr
Nature Chemistry Published:06 October 2025
DOI:https://doi.org/10.1038/s41557-025-01941-6
Abstract
Achieving efficient red and near-infrared (NIR) emission in boron cation-based emitters remains challenging owing to their intrinsic instability, strong electrophilicity of the boron centre and pronounced non-radiative decay governed by the energy gap law. Here we report a family of air- and moisture-stable carbodicarbene (CDC)-borabenzo[c]anthanthrenium ions exhibiting solid-state red-to-NIR luminescence (maximum emission wavelength up to 730 nm) with competitive quantum yields. Crystallographic, photophysical and computational analyses reveal that the CDC ligand plays a dual role by electronically stabilizing the boron centre and directing ion-pair assembly via charge localization, thereby modulating exciton coupling and aggregate-state emission. These cationic π-extended boron frameworks represent rare examples of monoboron-doped luminophores displaying deep-red-to-NIR emission. Our findings highlight that the combination of π-extension, a charge-directing CDC ligand and ion-pair assembly constitutes an effective strategy to access efficient red/NIR emitters, providing guiding principles for the design of functional long-wavelength emitting main-group materials.
空気安定性熱発光カルボジカルベン-ボラフルオレニウムイオン Air-Stable Thermoluminescent Carbodicarbene-Borafluorenium Ions
Kimberly K. Hollister,Andrew Molino,Grace Breiner,Jacob E. Walley,Kelsie E. Wentz,Ashley M. Conley,Diane A. Dickie,David J. D. Wilson,and Robert J. Gilliard Jr.
Journal of the American Chemical Society Published: January 4, 2022
DOI:https://doi.org/10.1021/jacs.1c11861
Abstract
Borenium ions, originally synthesized as fundamentally important laboratory curiosities, have attracted significant attention due to their applications in catalysis and frustrated Lewis pair chemistry. However, investigations of the materials properties of these types of compounds are exceptionally rare. Herein, we report the synthesis, molecular structures, and optical properties of a new class of air-stable borenium ions, stabilized by the strongly donating carbodicarbene (CDC) ligand (2, 3, 6). Notably, CDC-borafluorenium ions exhibit thermoluminescence in solution, a result of a twisted intramolecular charge transfer process. The temperature responsiveness, which is observable by the naked eye, is assessed over a 20 to −60 °C range. Significantly, compound 2 emits white light at lower temperatures. In the solid state, these borocations exhibit increased quantum yields due to aggregation-induced emission. CDC-borafluorenium ions with two different counteranions (Br–, BPh4–) were investigated to evaluate the effect of anion size on the solution and solid-state optical properties. In addition, CDCs containing both symmetrical and unsymmetrical N-heterocycles (bis(1-isopropyl-3-methylbenzimidazol-2-ylidene)methane and bis(1,3-dimethyl-1,3-dihydro-2H-benzo[d]imidazol-2-ylidene)methane) were tested to understand the implications of free rotation about the CDC ligand carbon–carbon bonds. The experimental work is complemented by a comprehensive theoretical analysis of the excited-state dynamics.