2025-06-30 東京科学大学
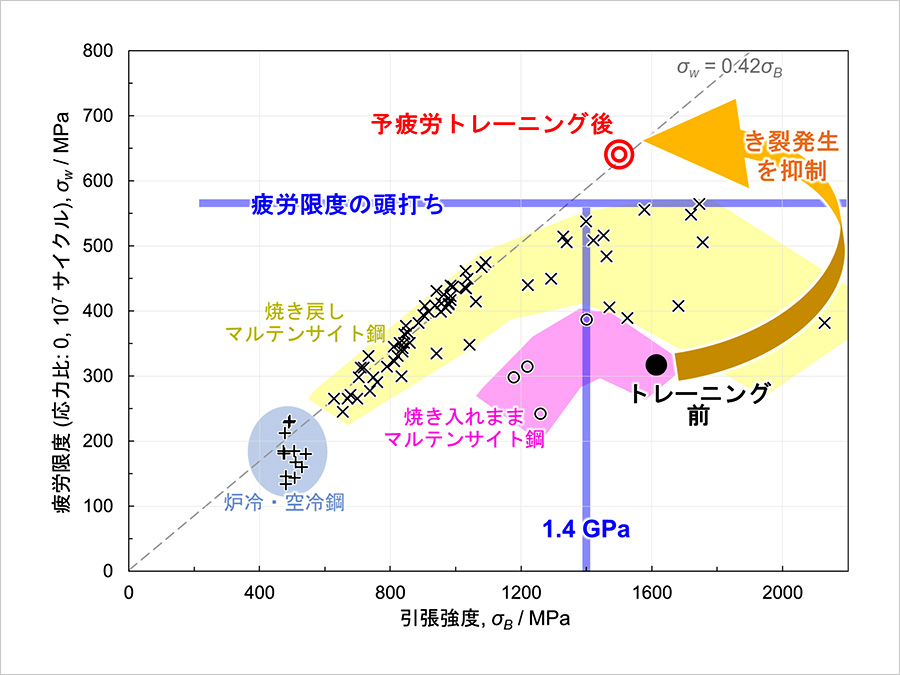
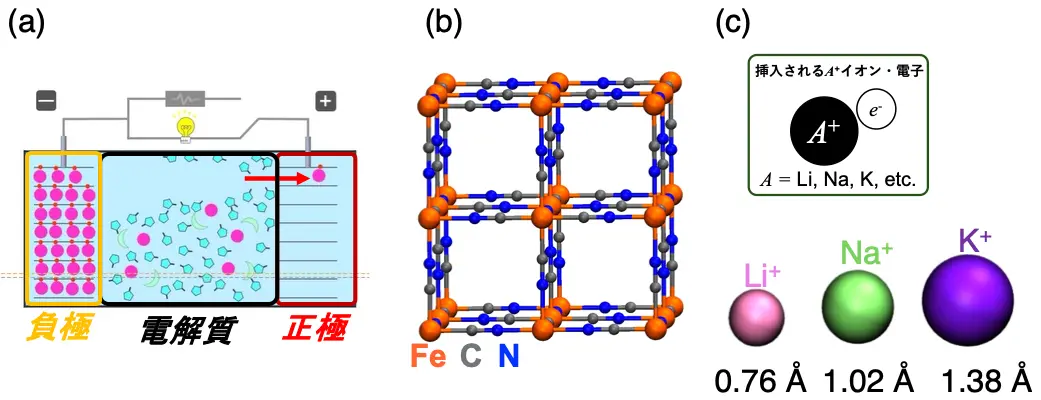 図1.(a)イオン二次電池の模式図。負極から正極へと、リチウムイオンなどのプラスの電荷を帯びたイオン(濃いピンク色の丸)が移動することで、電流が流れる。(b)正極材料の一種であるプルシアンブルー(PB)結晶。(c)PB結晶の孔の中で拡散するA+(= Li+、Na+、K+)イオンとそのサイズの比。
図1.(a)イオン二次電池の模式図。負極から正極へと、リチウムイオンなどのプラスの電荷を帯びたイオン(濃いピンク色の丸)が移動することで、電流が流れる。(b)正極材料の一種であるプルシアンブルー(PB)結晶。(c)PB結晶の孔の中で拡散するA+(= Li+、Na+、K+)イオンとそのサイズの比。
<関連情報>
- https://www.isct.ac.jp/ja/news/9wryndf41fk8
- https://www.isct.ac.jp/plugins/cms/component_download_file.php?type=2&pageId=&contentsId=1&contentsDataId=1781&prevId=&key=77a571f2db146b5967b9fe6af8208ca1.pdf
- https://pubs.acs.org/doi/10.1021/jacs.5c05274
無水Fe系プルシアンブルー正極におけるLi+、Na+、K+イオンの相異なる拡散機構 Dissimilar Diffusion Mechanisms of Li+, Na+, and K+ Ions in Anhydrous Fe-Based Prussian Blue Cathode
Dan Ito,Seong-Hoon Jang,Hideo Ando,Toshiyuki Momma,and Yoshitaka Tateyama
Journal of the American Chemical Society Published June 30, 2025
DOI:https://doi.org/10.1021/jacs.5c05274
Abstract
Prussian Blue (PB, AFe[Fe(CN)6], where A = Li, Na, K, etc.), a three-dimensional (3D) metal–organic framework (MOF), emerges as a promising cathode material, particularly for next-generation Na- and K-ion batteries. However, the microscopic occupation positions and diffusion behaviors of A+ ions in the unit cell have been inadequately elucidated. This study systematically compares the diffusion mechanisms of multiple Li+, Na+, and K+ ions using density functional theory calculations. We clarified the new stable occupation sites for Li+ and Na+ ions: the face-centered (FC) 24d and off-FC 48g sites, respectively. The smaller ionic radii of Li+ and Na+ ions contribute to their enhanced Coulombic attractions from CN– anions. Li+ ions are more self-diffusive than Na+ at high temperatures; however, at room temperature, Na+ ions have comparable self-diffusivities and lower activation energies than Li+ ions. This is attributed to the smaller tilting of [Fe(CN)6]-octahedra induced by Na+ ions’ transfers, resulting in a shallower potential energy landscape than for Li+ ions. These results demonstrated that the anhydrous Fe-based pristine PB crystal is an excellent Na+-ion conductor. Meanwhile, K+ ions prefer the conventional body center (8c site) and exhibit negligible self-diffusivities without anionic defects. Surprisingly, they show anisotropic diffusion along anion vacancy channels in the defective crystal, in contrast with the isotropic pathways for Li+ and Na+ ions. These findings update the fundamental chemistry of the diffusivity correlation with the electronic orbital interactions and framework distortion within general MOF materials.