2025-05-26 東京大学
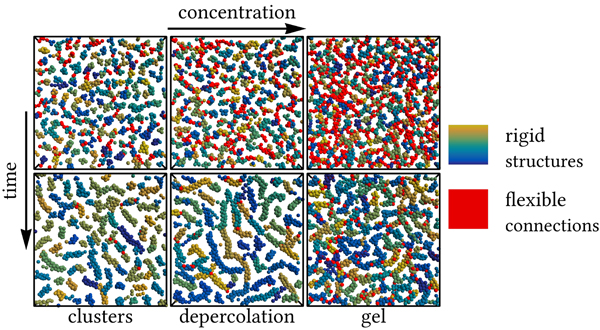
荷電コロイドの相分離の様子
<関連情報>
- https://www.rcast.u-tokyo.ac.jp/ja/news/release/20250526.html
- https://pubs.acs.org/doi/10.1021/acsnano.5c03244
非平衡コロイドゲル化における競合相互作用の影響を解明する Unraveling the Impact of Competing Interactions on Nonequilibrium Colloidal Gelation
Joeri Opdam,Michio Tateno,and Hajime Tanaka
ACS Nano Published: May 25, 2025
DOI:https://doi.org/10.1021/acsnano.5c03244
Abstract
Competing interactions stabilize exotic mesoscopic structures, yet the microscopic mechanisms by which they influence nonequilibrium processes leading to disordered states remain largely unexplored, despite their critical role in self-assembly across a range of nanomaterials and biological systems. Here, we numerically investigate the structural evolution in charged colloidal model systems, where short-range attractions and long-range repulsions compete. We reveal that these two interaction scales drive sequential ordering within clusters, from tetrahedra motifs to linear aggregates with chiral order. This process disrupts early stage percolated networks, resulting in reentrant behavior─a dynamic transition from disordered clusters to network to chiral rigid clusters. On the other hand, the cluster-elastic network boundary in the final state is governed by isostatic percolation, which slows structural rearrangements, preserves branching points, and sustains a long-lived network. The resulting structure consists of rigid Bernal spiral-like branches connected through flexible branching points lacking order. These insights advance our microscopic understanding of out-of-equilibrium ordering driven by competing interactions, especially phenomena such as temporally delayed frustration reflecting different length scales of competing interactions. The mechanisms identified here may play a crucial role in mesoscale self-organization across soft materials, from nanoparticle assemblies to biological gels and cytoskeletal networks. Understanding how competing interactions regulate structure and dynamics could guide the design of adaptive materials with tunable mechanical properties and offer valuable insights into biological processes such as cytoplasmic organization and cellular scaffolding.