2025-05-09 北海道大学,科学技術振興機構
<関連情報>
- https://www.hokudai.ac.jp/news/2025/05/post-1870.html
- https://www.hokudai.ac.jp/news/pdf/250509_pr.pdf
- https://www.nature.com/articles/s41467-025-59317-w
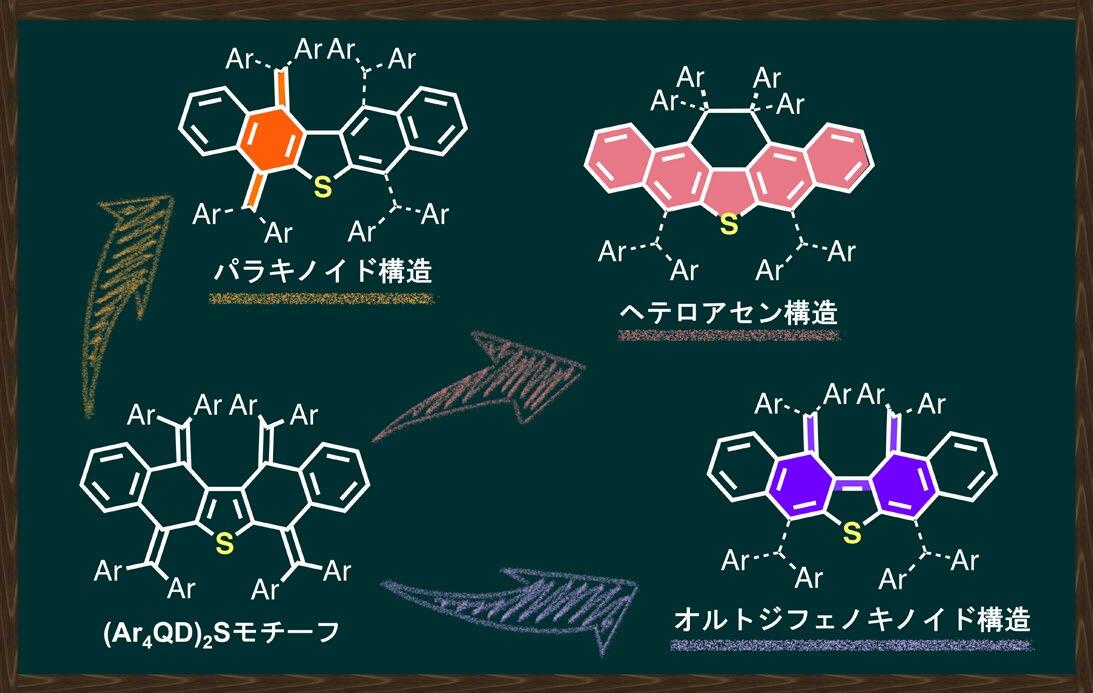
パラ-キノイド、σ結合、オルト-ジフェノキノイド構造の創出を実現する酸化還元刺激による多様な構造変換 Diverse redox-mediated transformations to realize the para-quinoid, σ-bond, and ortho-diphenoquinoid forms
Takashi Harimoto,Moto Kikuchi,Takanori Suzuki & Yusuke Ishigaki
Nature Communications Published:08 May 2025
DOI:https://doi.org/10.1038/s41467-025-59317-w
Abstract
π-Electron systems with multiple redox-active units have attracted attention in various fields due to their potential applications. However, the design strategy remains elusive to selectively synthesize the diverse molecular structures of redox-convertible species. In this study, covalently linked quinodimethane derivatives with a sulfur bridge [(Ar4QD)2S] were designed as redox-active motifs that can be converted into three different geometries via redox reaction. Here we show that the favored geometry of the corresponding redox states of (Ar4QD)2S can be precisely controlled by adjusting the steric bulk of the substituents on the aryl group to change the proximity of the quinodimethane units. Notably, this redox-mediated strategy also leads to the isolation and structural determination of the missing link with an o-diphenoquinoid structure, a diphenoquinoid isomer whose isolation had remained elusive for almost a century. Thus, this study provides a method that allows the modulation/control of electronically and/or thermodynamically stable structures, as well as their electronic and spectroscopic properties.