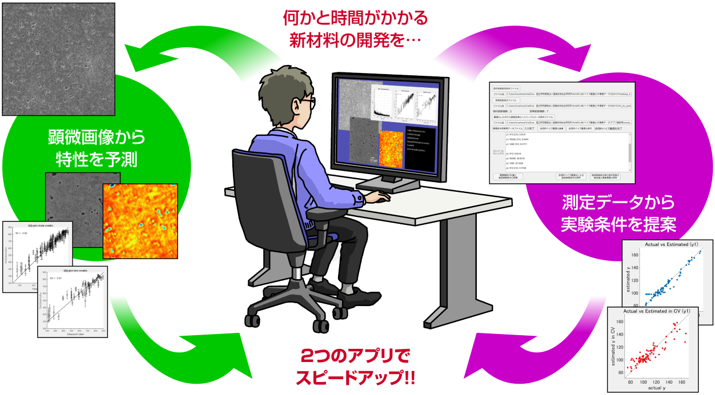
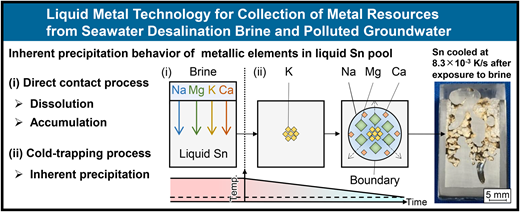
2025-04-23 名古屋大学
<関連情報>
- https://www.nagoya-u.ac.jp/researchinfo/result/2025/04/post-810.html
- https://www.nagoya-u.ac.jp/researchinfo/result/upload_images/20250423_sci.pdf
- https://pubs.rsc.org/en/content/articlelanding/2025/sc/d5sc01415h
スルホニウム化による多環芳香族化合物の機能化と可溶化 Functionalization and solubilization of polycyclic aromatic compounds by sulfoniumization
Johannes E. Erchinger, Tsubasa Okumura, Kanami Nakata, Daisuke Shimizu, Constanstin G. Daniliuc, Kazuma Amaike, Frank Glorius, Kenichiro Itami and Hideto Ito
Chemical Science Published:11 Apr 2025
DOI:https://doi.org/10.1039/D5SC01415H
Abstract
Despite their unique physical properties and diverse applications in materials science, poor solubility of polycyclic aromatic hydrocarbons (PAHs) limits further fine-tuning and investigation of these systems. Herein, we report a sulfoniumization strategy to solubilize and functionalize a diverse range of PAHs in a one-step protocol using a triethylene glycol ether-substituted diaryl sulfoxide. While mono-sulfoniumization is generally observed, modification of the reaction conditions to favor bis-sulfoniumization is shown. The downstream applicability of the resulting PAH sulfonium salts is validated through a series of post-functionalization reactions that include C–C and C–heteroatom bond formation, while their application in annulative π-extension (APEX) is showcased by the synthesis of tetra-tert-butylquaterrylene from perylene. The red-shifted absorption and fluorescence, along with high water solubility of the PAH sulfonium salts, enable their application in bio-imaging, where they demonstrate selective mitochondrial staining without cytotoxicity.