2025-033-26 デラウェア大学(UD)
<関連情報>
- https://www.udel.edu/udaily/2025/march/mareks-disease-chickens-nigeria-research-mark-parcells/
- https://www.mdpi.com/1999-4915/17/1/56
- https://www.sciencedirect.com/science/article/abs/pii/S0378113504002160
ナイジェリアのマレック病ウイルス(MDV)野外分離株のMeq遺伝子には、ヨーロッパとアメリカの高ウイルス株と共通の変異がある The Meq Genes of Nigerian Marek’s Disease Virus (MDV) Field Isolates Contain Mutations Common to Both European and US High Virulence Strains
Joseph N. Patria,Luka Jwander,Ifeoma Mbachu,Levi Parcells,Brian Ladman,Jakob Trimpert,Benedikt B. Kaufer,Phaedra Tavlarides-Hontz
Viruses Published: 31 December 2024
DOI:https://doi.org/10.3390/v17010056
Abstract
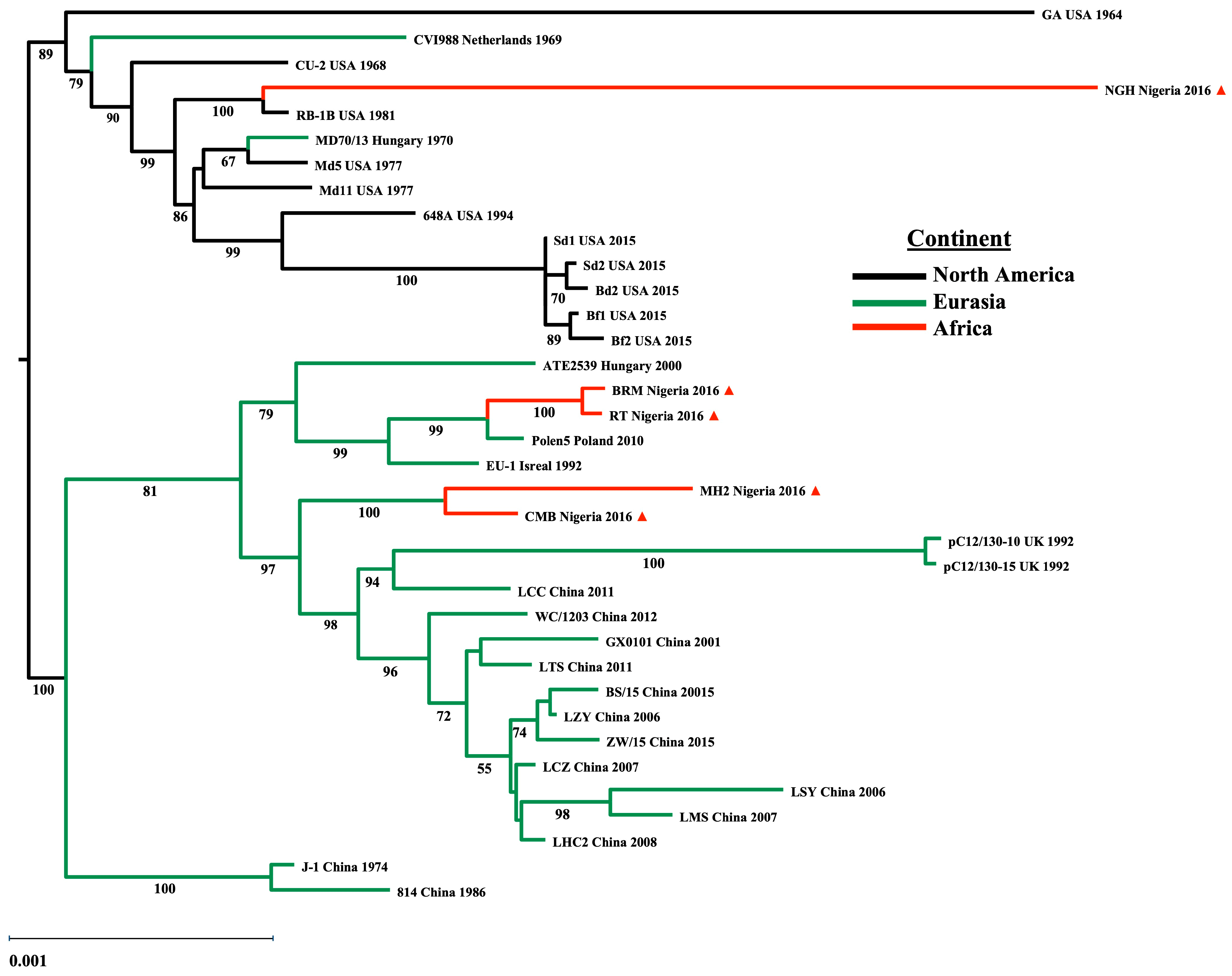
Background: Marek’s disease (MD) is a pathology affecting chickens caused by Marek’s disease virus (MDV), an acute transforming alphaherpesvirus of the genus Mardivirus. MD is characterized by paralysis, immune suppression, and the rapid formation of T-cell (primarily CD4+) lymphomas. Over the last 50 years, losses due to MDV infection have been controlled worldwide through vaccination; however, these live-attenuated vaccines are non-sterilizing and potentially contributed to the virulence evolution of MDV field strains. Mutations common to field strains that can overcome vaccine protection were identified in the C-terminal proline-rich repeats of the oncoprotein Meq (Marek’s EcoRI-Q-encoded protein). These mutations in meq have been found to be distinct to their region of origin, with high virulence strains obtained in Europe differing from those having evolved in the US. The present work reports on meq mutations identified in MDV field strains in Nigeria, arising at farms employing different vaccination practices. Materials and Methods: DNA was isolated from FTA cards obtained at 12 farms affected by increased MD in the Plateau State, Nigeria. These sequences included partial whole genomes as well as targeted sequences of the meq oncogenes from these strains. Several of the meq genes were cloned for expression and their localization ability to interact with the chicken NF-IL3 protein, a putative Meq dimerization partner, were assessed. Results: Sequence analysis of the meq genes from these Nigerian field strains revealed an RB1B-like lineage co-circulating with a European Polen5-like lineage, as well as recombinants harboring a combination of these mutations. In a number of these isolates, Meq mutations accumulated in both N-terminal and C-terminal domains. Discussion: Our data, suggest a direct effect of the vaccine strategy on the selection of Meq mutations. Moreover, we posit the evolution of the next higher level of virulence MDVs, a very virulent plus plus pathotype (vv++).
マレック病ウイルス(MDV)の糖タンパク質、溶菌抗原pp38、形質転換抗原Meqをコードする遺伝子の比較解析:Meq変異と病原性の高いMDVとの関連性 Comparative analysis of Marek’s disease virus (MDV) glycoprotein-, lytic antigen pp38- and transformation antigen Meq-encoding genes: association of meq mutations with MDVs of high virulence
Christine E. Shamblin, Natalie Greene, Vaithilingaraja Arumugaswami, Robert L. Dienglewicz, Mark S. Parcells
Veterinary Microbiology Available online: 1 August 2004
DOI:https://doi.org/10.1016/j.vetmic.2004.06.007
Abstract
Marek’s disease (MD) is a highly contagious lymphoproliferative and demyelinating disorder of chickens. MD is caused by Marek’s disease virus (MDV), a cell-associated, acute-transforming alphaherpesvirus. For three decades, losses to the poultry industry due to MD have been greatly limited through the use of live vaccines. MDV vaccine strains are comprised of antigenically related, apathogenic MDVs originally isolated from chickens (MDV-2), turkeys (herpesvirus of turkeys, HVT) or attenuated-oncogenic strains of MDV-1 (CVI-988). Since the inception of high-density poultry production and MD vaccination, there have been two discernible increases in the virulence of MDV field strains. Our objectives were to determine if common mutations in the major glycoprotein genes, a major lytic antigen phosphoprotein 38 (pp38) or a major latency/transformation antigen Meq (Marek’s EcoRI-Q-encoded protein) were associated with enhanced MDV virulence. To address this, we cloned and sequenced the major surface glycoprotein genes (gB, gC, gD, gE, gH, gI, and gL) of five MDV strains that were representative of the virulent (v), very virulent (vv) and very virulent plus (vv+) pathotypes of MDV. We found no consistent mutations in these genes that correlated strictly with virulence level. The glycoprotein genes most similar among MDV-1, MDV-2 and HVT (gB and gC, ∼81 and 75%, respectively) were among the most conserved across pathotype. We found mutations mapping to the putative signal cleavage site in the gL genes in four out of eleven vv+MDVs, but this mutation was also identified in one vvMDV (643P) indicating that it did not correlate with enhanced virulence. In further analysis of an additional 12 MDV strains, we found no gross polymorphism in any of the glycoprotein genes. Likewise, by PCR and RFLP analysis, we found no polymorphism at the locus encoding the pp38 gene, an early lytic-phase gene associated with MDV replication. In contrast, we found distinct mutations in the latency and transformation-associated Marek’s EcoRI-Q–encoded protein, Meq. In examination of the DNA and deduced amino acid sequence of meq genes from 26 MDV strains (9 m/vMDV, 5 vvMDV and 12 vv+MDVs), we found distinct polymorphism and point mutations that appeared to correlate with virulence. Although a complex trait like MDV virulence is likely to be multigenic, these data describe the first sets of mutations that appear to correlate with MDV virulence. Our conclusion is that since Meq is expressed primarily in the latent/transforming phase of MDV infection, and is not encoded by MDV-2 or HVT vaccine viruses, the evolution of MDV virulence may be due to selection on MDV-host cell interactions during latency and may not be mediated by the immune selection against virus lytic antigens such as the surface glycoproteins.