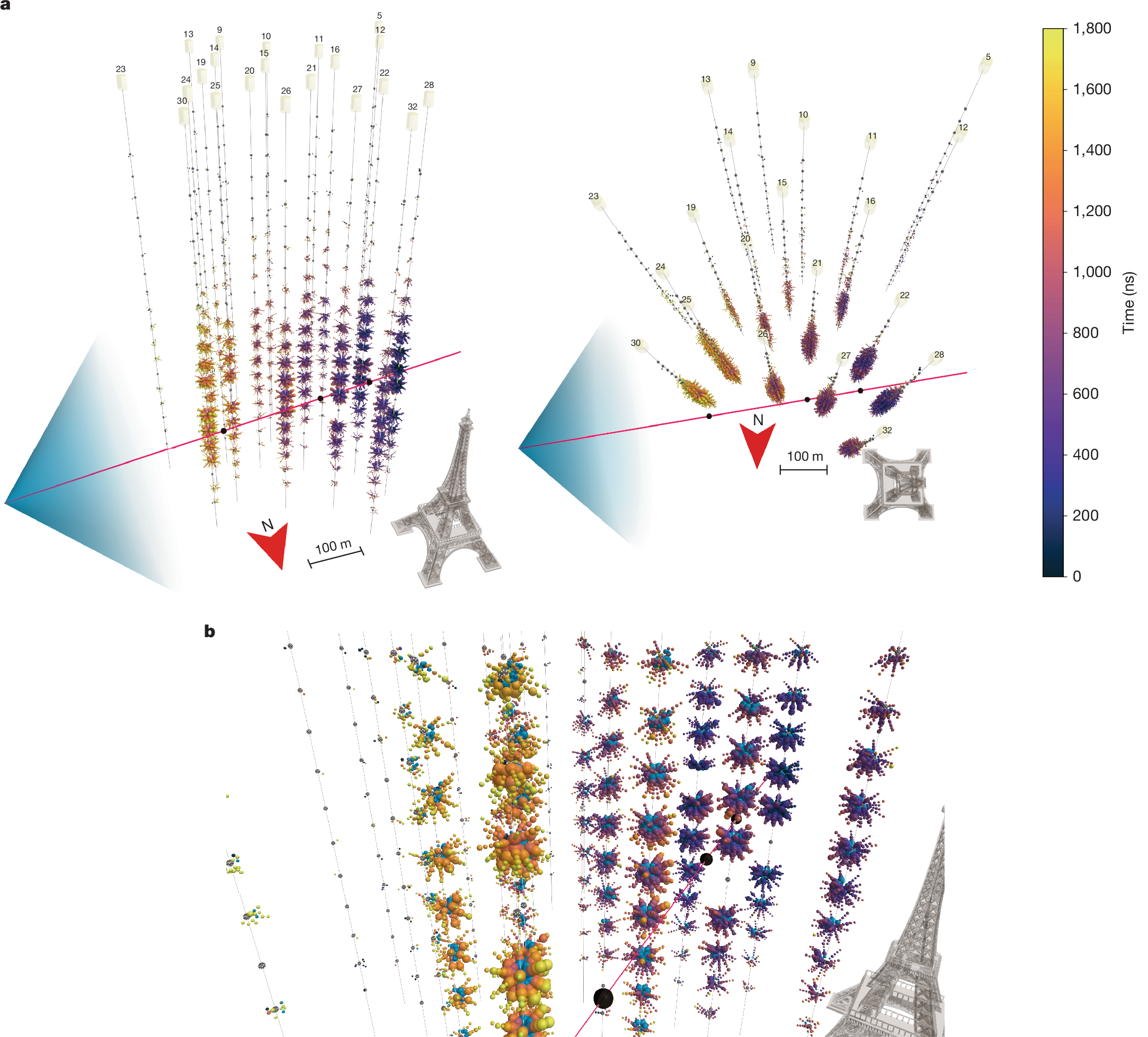
2025-02-20 タフツ大学

A computer-generated image showing single nickel (Ni) atoms embedded in silver, used “to enhance oxygen dissociation and enable efficient production of ethylene oxide, a commodity chemical,” says Charles Sykes. Image: Elizabeth Happel
<関連情報>
- https://now.tufts.edu/2025/02/20/researchers-discover-potentially-cleaner-way-make-important-chemical
- https://www.science.org/doi/10.1126/science.adt1213
ニッケルは銀上での選択的エチレンエポキシ化を促進する Nickel promotes selective ethylene epoxidation on silver
Anika Jalil, Elizabeth E. Happel, Laura Cramer, Adrian Hunt, […], and E. Charles H. Sykes
Science Published:20 Feb 2025
Editor’s summary
Traces of nickel can increase the selectivity of supported silver ethylene epoxidation catalysts to levels comparable to that of added alkyl chloride promoters. Theoretical studies by Jalil et al. showed that nickel dopants on silver could activate molecular oxygen, and surface science experiments showed that nickel could stabilize nucleophilic oxygen that would otherwise react unselectively. Parts per million addition of nickel enhanced selectivity by 25%, and added chlorine could also boost the effect of nickel by an additional 10%. —Phil Szuromi
Abstract
Over the last 80 years, chlorine (Cl) has been the primary promoter of the ethylene epoxidation reaction valued at ~40 billion USD per year, providing a ~25% selectivity increase over unpromoted silver (Ag) (~55%). Promoters such as cesium, rhenium, and molybdenum each add a few percent of selectivity enhancements to achieve 90% overall, but their codependence on Cl makes optimizing and understanding their function complex. We took a theory-guided, single-atom alloy approach to identify nickel (Ni) as a dopant in Ag that can facilitate selective oxidation by activating molecular oxygen (O2) without binding oxygen (O) too strongly. Surface science experiments confirmed the facile adsorption/desorption of O2 on NiAg, as well as demonstrating that Ni serves to stabilize unselective nucleophilic oxygen. Supported Ag catalyst studies revealed that the addition of Ni in a 1:200 Ni to Ag atomic ratio provides a ~25% selectivity increase without the need for Cl co-flow and acts cooperatively with Cl, resulting in a further 10% initial increase in selectivity.