2023-08-31 ペンシルベニア州立大学(PennState)
◆Penn Stateの研究チームは、この現象がどのように起こり、なぜ起こるのかを初めて定量化し、水素の活性化と貯蔵の可能性を開く重要な研究成果を得ました。水素スピルオーバーは、クリーンな燃料としての利用や水素貯蔵などの新たな応用に道を開く可能性があります。
<関連情報>
- https://www.psu.edu/news/engineering/story/striking-gold-molecular-mystery-solution-potential-clean-energy/
- https://www.nature.com/articles/s41929-023-00996-3
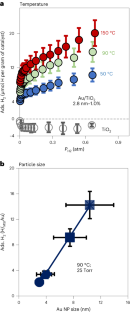
金/酸化チタン触媒におけるH2のエントロピー駆動型吸着とスピルオーバーにおける表面ヒドロキシルの役割 The role of surface hydroxyls in the entropy-driven adsorption and spillover of H2 on Au/TiO2 catalysts
Akbar Mahdavi-Shakib,Todd N. Whittaker,Tae Yong Yun,K. B. Sravan Kumar,Lauren C. Rich,Shengguang Wang,Robert M. Rioux,Lars C. Grabow &Bert D. Chandler
Nature Catalysis Published:10 August 2023
DOI:https://doi.org/10.1038/s41929-023-00996-3
Abstract
Hydrogen spillover involves the migration of H atom equivalents from metal nanoparticles to a support. While well documented, H spillover is poorly understood and largely unquantified. Here we measure weak, reversible H2 adsorption on Au/TiO2 catalysts, and extract the surface concentration of spilled-over hydrogen. The spillover species (H*) is best described as a loosely coupled proton/electron pair distributed across the titania surface hydroxyls. In stark contrast to traditional gas adsorption systems, H* adsorption increases with temperature. This unexpected adsorption behaviour has two origins. First, entropically favourable adsorption results from high proton mobility and configurational surface entropy. Second, the number of spillover sites increases with temperature, due to increasing hydroxyl acid–base equilibrium constants. Increased H* adsorption correlates with the associated changes in titania surface zwitterion concentration. This study provides a quantitative assessment of how hydroxyl surface chemistry impacts spillover thermodynamics, and contributes to the general understanding of spillover phenomena.