2025-09-05 東京大学
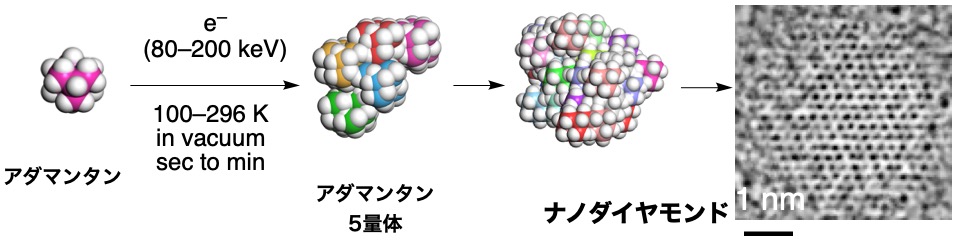
アダマンタンからのナノダイヤモンド合成
<関連情報>
アマンタンC–H結合の電子線活性化による迅速・低温ナノダイヤモンド形成 Rapid, low-temperature nanodiamond formation by electron-beam activation of adamantane C–H bonds
Jiarui Fu, Takayuki Nakamuro, and Eiichi Nakamura
Science Published:4 Sep 2025
DOI:https://doi.org/10.1126/science.adw2025
Editor’s summary
Adamantane can adopt the same tetrahedral carbon skeleton as diamond. Fu et al. found that electron-beam irradiation of adamantane crystallites in a transmission electron microscope at low temperatures (296 to 100 K) and under vacuum conditions led to the activation of carbon-hydrogen bonds. These conditions enabled the formation of carbon-carbon bonds to create a diamond lattice. —Phil Szuromi
Abstract
Diamond and adamantane (Ad) share a Td-symmetric carbon skeleton, but converting Ad to diamond has been challenging because it requires selective carbon-hydrogen (C–H) bond cleavage and monomer assembly into a diamond lattice. Our approach differs from the conventional high-temperature, high-pressure diamond syntheses. We electron-irradiated Ad submicrocrystals at 80 to 200 kilo–electron volts and 100 to 296 kelvin in vacuum for tens of seconds. This process yielded defect-free nanodiamonds (NDs) of cubic crystal structure, accompanied by hydrogen gas evolution. Time-resolved transmission electron microscopy revealed the initial formation of Ad oligomers transforming into spherical NDs. A sizable kinetic isotope effect indicates that C–H cleavage was rate-determining, and other hydrocarbons tested failed to form NDs.