2025-05-20 パシフィック・ノースウェスト国立研究所(PNNL)

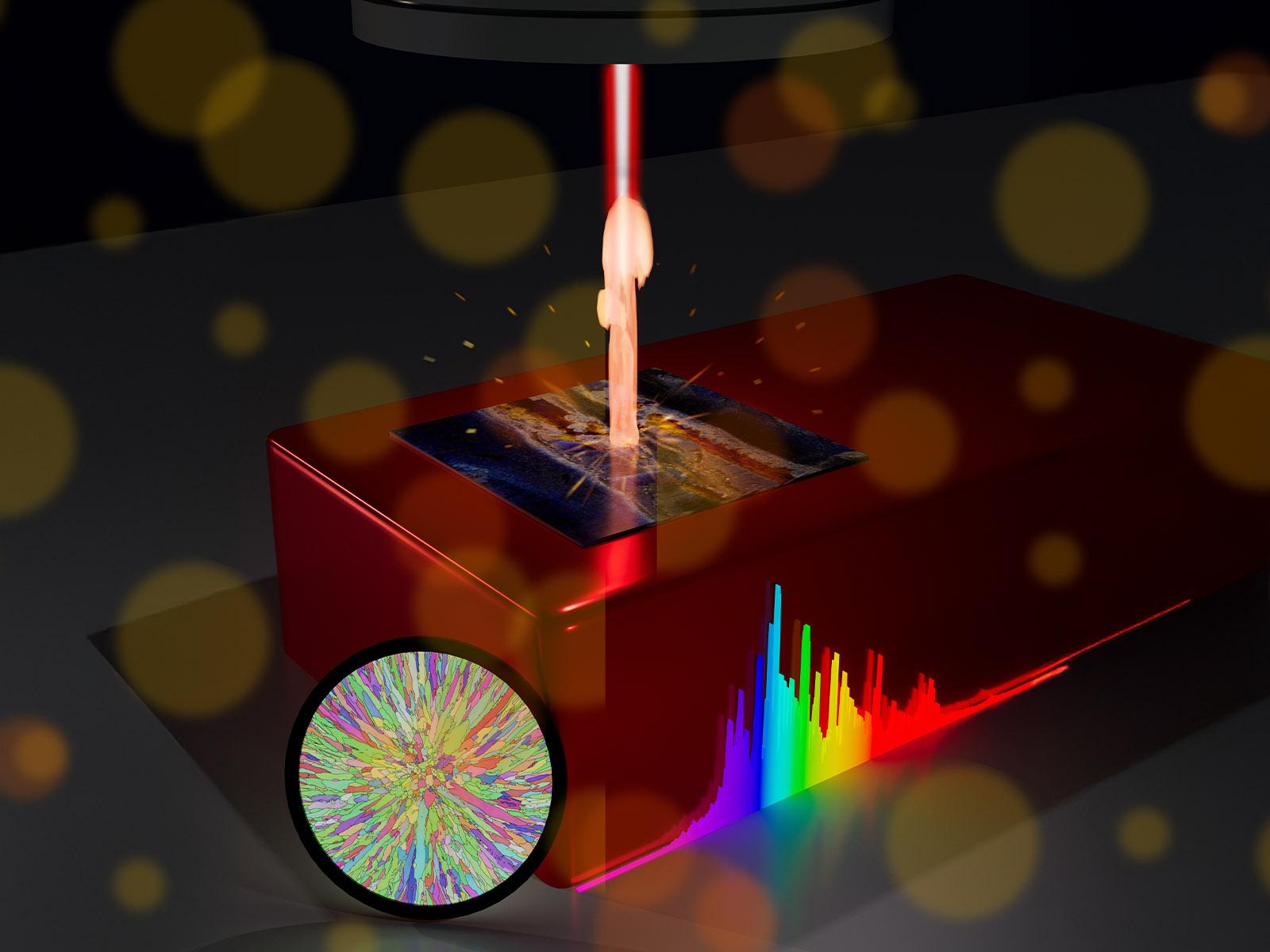
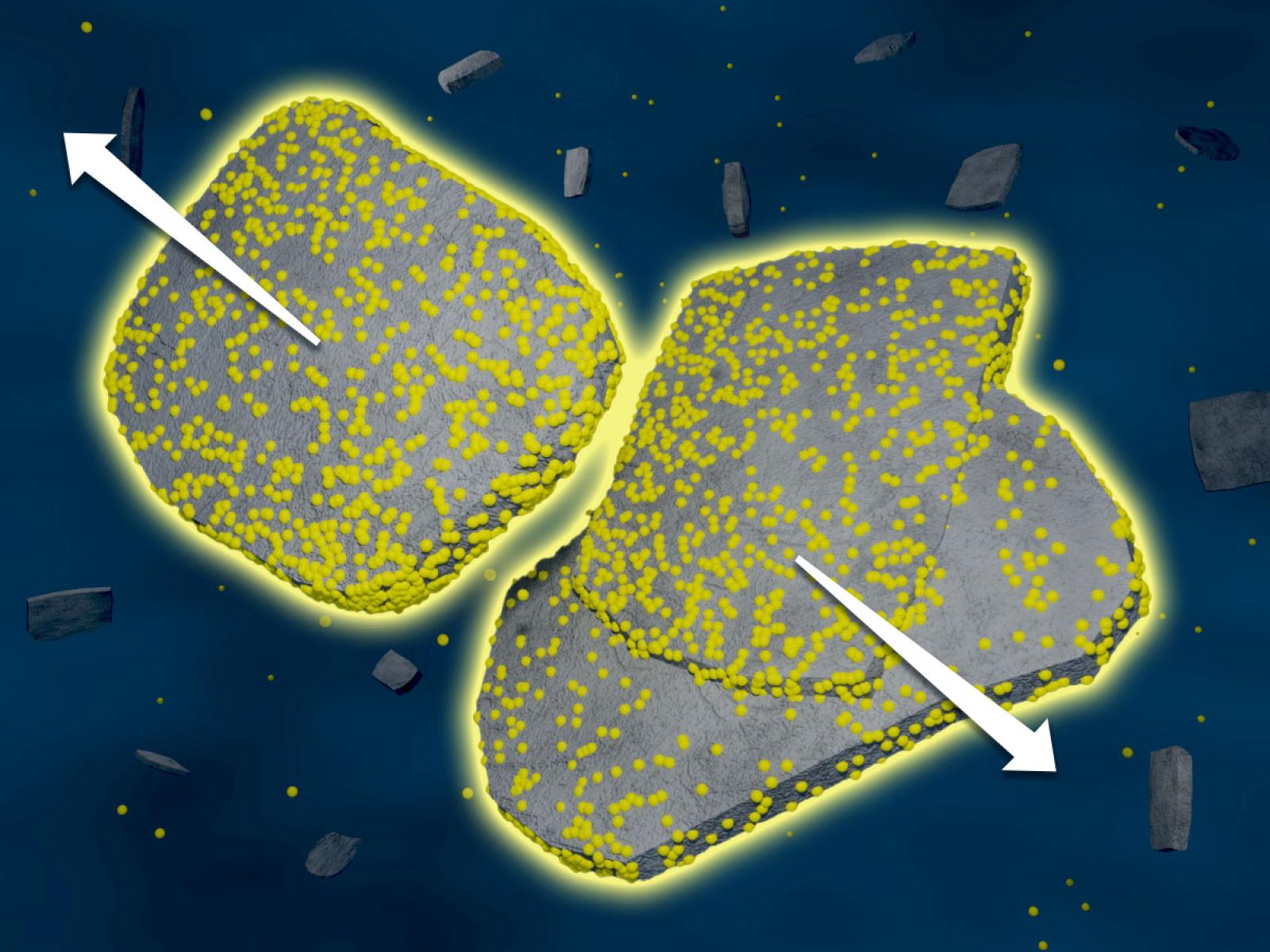
An energy-efficient, novel reaction–diffusion process successfully separates iron, dysprosium, and neodymium without relying on traditional ligands, membranes, or resins. (Image by Qingpu Wang | Pacific Northwest National Laboratory)
<関連情報>
- https://www.pnnl.gov/publications/enabling-urban-mining-critical-minerals-domestic-electronic-waste
- https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/cssc.202402372?af=R
反応拡散カップリングによる模擬電子廃棄物からの重要鉱物の選択的回収 Selective Recovery of Critical Minerals from Simulated Electronic Wastes Via Reaction-Diffusion Coupling
Dr. Qingpu Wang, Dr. Yucheng Fu, Dr. Erin A. Miller, Dr. Duo Song, Philip J. Brahana, Dr. Andrew Ritchhart, Dr. Zhijie Xu, Dr. Grant E. Johnson, Prof. Dr. Bhuvnesh Bharti, Dr. Maria L. Sushko, Dr. Elias Nakouzi
ChemSusChem Published: 05 February 2025
DOI:https://doi.org/10.1002/cssc.202402372
Abstract
Atom- and energy-efficient chemical separations are urgently needed to meet the surging demand for critical materials that has strained supply chains and threatened environmental damage. In this study, we used reaction-diffusion coupling to separate iron, neodymium, and dysprosium ions from model feedstocks of permanent magnets, which are typically found in electronic wastes. Feedstock solutions were placed in contact with a hydrogel loaded with potassium hydroxide and/or dibutyl phosphate, resulting in complex precipitation patterns as the various metal ions diffused into the reaction medium. Specifically, we observed the precipitation of up to 40 mM of iron from the feedstock, followed by the enrichment of 73 % dysprosium, and the extraction of >95 % neodymium product at a further distance from the solution-gel interface. We designed a series of experiments and simulations to determine the relevant ion diffusivities, DNd=5.4×10−10 and DDy=5.1×10−10 m2/s, and precipitation rates, kNd =1.0×10−5 and kDy=5.0×10−3 m9 mol−3 s−1, which enabled a numerical model to be established for predicting the distribution of products in the reaction medium. Our proof-of-concept study validates reaction-diffusion coupling as an effective and versatile approach for critical materials separations, without relying on ligands, membranes, resins, or other specialty chemicals.