2024-03-27 バッファロー大学(UB)
<関連情報>
- https://www.buffalo.edu/news/releases/2024/03/methane-capture-catalysts.html
- https://www.nature.com/articles/s41467-024-45413-w
熱力学への挑戦:単相ナノセラミックにおける非混和性元素の結合 Challenging thermodynamics: combining immiscible elements in a single-phase nano-ceramic
Shuo Liu,Chaochao Dun,Qike Jiang,Zhengxi Xuan,Feipeng Yang,Jinghua Guo,Jeffrey J. Urban & Mark T. Swihart
Nature Communications Published:07 February 2024
DOI:https://doi.org/10.1038/s41467-024-45413-w
Abstract
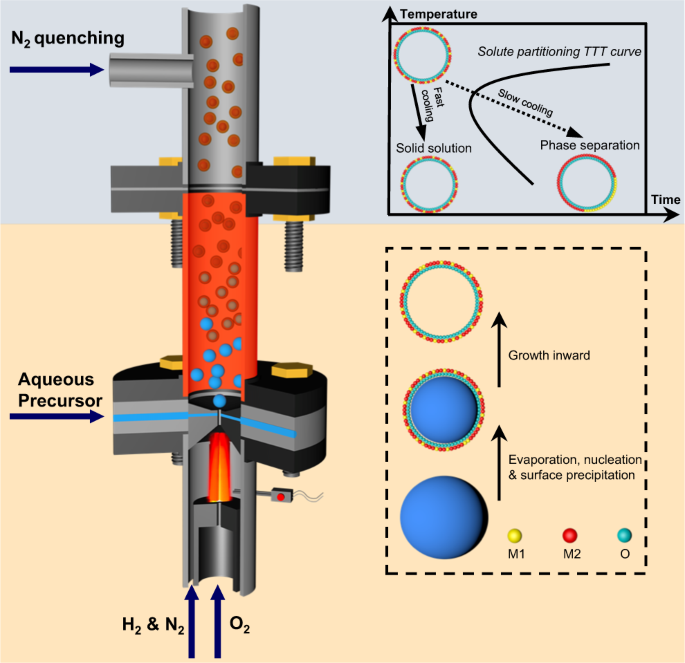
The Hume-Rothery rules governing solid-state miscibility limit the compositional space for new inorganic material discovery. Here, we report a non-equilibrium, one-step, and scalable flame synthesis method to overcome thermodynamic limits and incorporate immiscible elements into single phase ceramic nanoshells. Starting from prototype examples including (NiMg)O, (NiAl)Ox, and (NiZr)Ox, we then extend this method to a broad range of Ni-containing ceramic solid solutions, and finally to general binary combinations of elements. Furthermore, we report an “encapsulated exsolution” phenomenon observed upon reducing the metastable porous (Ni0.07Al0.93)Ox to create ultra-stable Ni nanoparticles embedded within the walls of porous Al2O3 nanoshells. This nanoconfined structure demonstrated high sintering resistance during 640 h of catalysis of CO2 reforming of methane, maintaining constant 96% CH4 and CO2 conversion at 800 °C and dramatically outperforming conventional catalysts. Our findings could greatly expand opportunities to develop novel inorganic energy, structural, and functional materials.



