2024-02-23 インペリアル・カレッジ・ロンドン(ICL)
<関連情報>
- https://www.imperial.ac.uk/news/251633/migraine-research-beating-human-hearts-news/
- https://www.nature.com/articles/s41557-024-01450-y
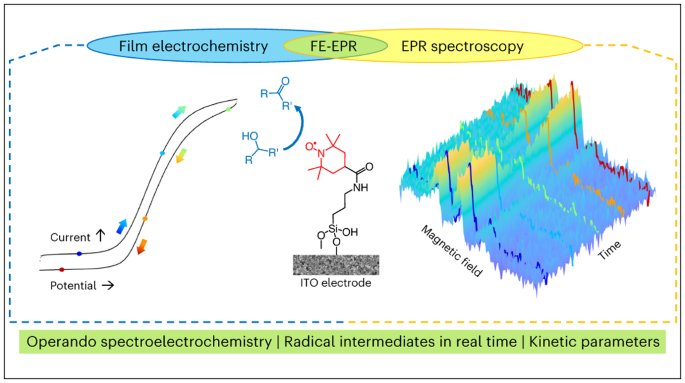
表面固定化触媒のラジカル中間体を追跡するオペランドフィルム電気化学EPR分光法 Operando film-electrochemical EPR spectroscopy tracks radical intermediates in surface-immobilized catalysts
Maryam Seif-Eddine,Samuel J. Cobb,Yunfei Dang,Kaltum Abdiaziz,Mark A. Bajada,Erwin Reisner & Maxie M. Roessler
Nature Chemistry Published:14 February 2024
DOI:https://doi.org/10.1038/s41557-024-01450-y
Abstract
The development of surface-immobilized molecular redox catalysts is an emerging research field with promising applications in sustainable chemistry. In electrocatalysis, paramagnetic species are often key intermediates in the mechanistic cycle but are inherently difficult to detect and follow by conventional in situ techniques. We report a new method, operando film-electrochemical electron paramagnetic resonance spectroscopy (FE-EPR), which enables mechanistic studies of surface-immobilized electrocatalysts. This technique enables radicals formed during redox reactions to be followed in real time under flow conditions, at room temperature and in aqueous solution. Detailed insight into surface-immobilized catalysts, as exemplified here through alcohol oxidation catalysis by a surface-immobilized nitroxide, is possible by detecting active-site paramagnetic species sensitively and quantitatively operando, thereby enabling resolution of the reaction kinetics. Our finding that the surface electron-transfer rate, which is of the same order of magnitude as the rate of catalysis (accessible from operando FE-EPR), limits catalytic efficiency has implications for the future design of better surface-immobilized catalysts.