2025-12-12 東京科学大学
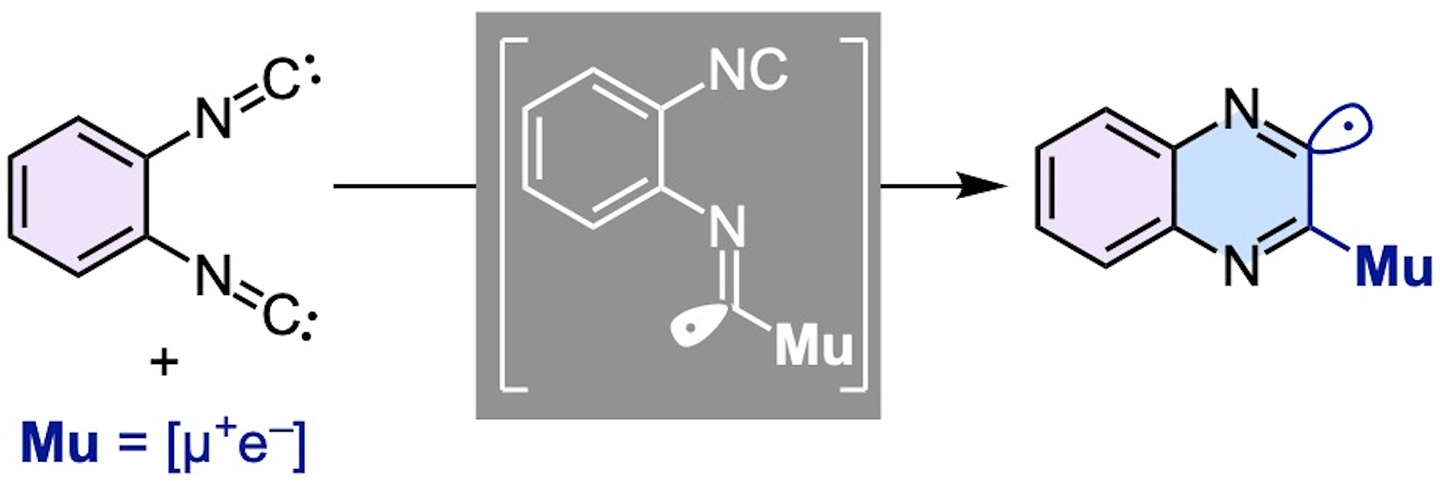
図1. 1,2-ジイソシアノベンゼン(左)にミュオニウムが付加して生成するイミドイルラジカル(中央)は速やかに環を形成してキノキサリニルラジカル(右)に変化する。
<関連情報>
- https://www.isct.ac.jp/ja/news/pxt5z1zprppn
- https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/chem.202503139
1,2-ジイソシアノベンゼンへのミュオニウム付加により生成したキノキサリニルラジカルの観察 Observation of Quinoxalinyl Radical Produced by Muonium Addition to 1,2-Diisocyanobenzene
Shigekazu Ito, Kazuki Iwami, Kenji M. Kojima, Iain McKenzie
Chemistry -A European Journal Published: 24 November 2025
DOI:https://doi.org/10.1002/chem.202503139
ABSTRACT
Radical isocyanide (isonitrile) insertion reactions have advanced considerably, yet the nature of the underlying paramagnetic intermediates remains poorly understood. We previously studied the isocyanide insertion of muonium (Mu), a light isotope of hydrogen, into 2-isocyano-1,1′-biphenyls by transverse field muon spin rotation (TF-µSR) spectroscopy and characterized the resulting radical, which was identified as an imidoyl radical. The cyclized product was not observed on the microsecond timescale. To gain deeper insight, we now report TF-µSR spectra of 1,2-diisocyano-3,4,5,6-tetramethylbenzene in both THF solution and the crystalline state. A single muoniated radical species was observed, displaying an unusually small muon hyperfine coupling constant (hfc). Guided by density functional theory (DFT) calculations, we identified this species as a quinoxalinyl radical, formed via intramolecular cyclization of the imidoyl intermediate. This observation implies that the imidoyl radical survives only on the nanosecond timescale before cyclization.