2025-04-29 中国科学院(CAS)
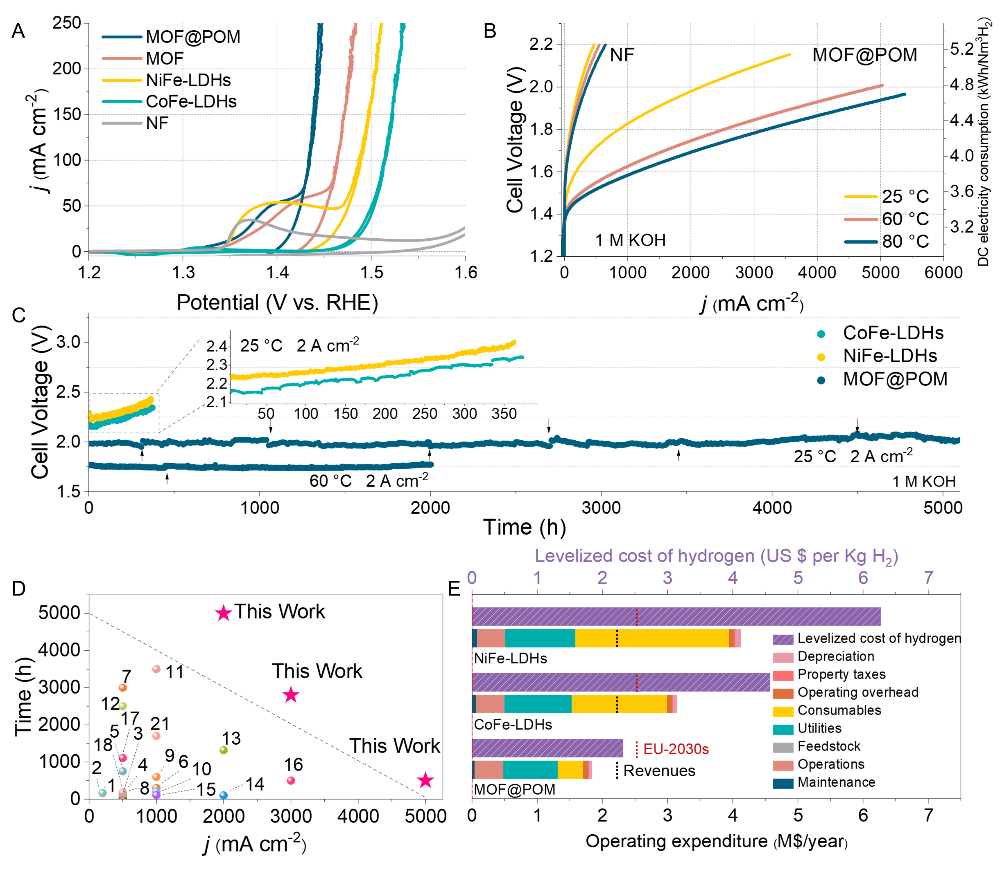 (A–C) Catalytic activity and operational stability of the MOF@POM superstructure under practical conditions. (Image by YAN Ya)
(A–C) Catalytic activity and operational stability of the MOF@POM superstructure under practical conditions. (Image by YAN Ya)
<関連情報>
- https://english.cas.cn/newsroom/research_news/chem/202504/t20250429_1042307.shtml
- https://www.science.org/doi/10.1126/science.ads1466
ポリオキソメタル化された有機金属骨格の超構造が安定した水の酸化を可能にする Polyoxometalated metal-organic framework superstructure for stable water oxidation
Kaihang Yue, Ruihu Lu, Mingbin Gao, Fei Song, […] , and Ya Yan
Science Published:24 Apr 2025
DOI:https://doi.org/10.1126/science.ads1466
Editor’s summary
Splitting water electrochemically is an appealing method for sustainable and environmentally friendly hydrogen production. However, current catalysts still lack the stability and activity to cost-effectively scale the process for the envisioned applications. Yue et al. report an earth-abundant multimetallic catalyst for the oxygen evolution side that exhibits high stability under alkaline conditions. The catalyst assembly combines an iron and cobalt metal organic framework with nickel and tungsten polyoxometalate clusters, which the authors posit facilitates efficient electron transfer in a stable structure. —Jake S. Yeston
Abstract
Stable, nonprecious catalysts are vital for large-scale alkaline water electrolysis. Here, we report a grafted superstructure, MOF@POM, formed by self-assembling a metal-organic framework (MOF) with polyoxometalate (POM). In situ electrochemical transformation converts MOF into active metal (oxy)hydroxides to produce a catalyst with a low overpotential of 178 millivolts at 10 milliamperes per square centimeter in alkaline electrolyte. An anion exchange membrane water electrolyzer incorporating this catalyst achieves 3 amperes per square centimeter at 1.78 volts at 80°C and stable operation at 2 amperes per square centimeter for 5140 hours at room temperature. In situ electrochemical spectroscopy and theoretical studies reveal that the synergistic interactions between metal atoms create a fast electron-transfer channel from catalytic iron and cobalt sites, nickel, and tungsten in the polyoxometalate to the electrode, stabilizing the metal sites and preventing dissolution.