2025-03-25 理化学研究所
<関連情報>
- https://www.riken.jp/press/2025/20250325_2/index.html
- https://www.nature.com/articles/s44160-025-00771-1
鉄触媒による有機ナトリウム化合物の直接カップリング Iron-catalysed direct coupling of organosodium compounds
Ikko Takahashi,Andreu Tortajada,David E. Anderson,Laurean Ilies,Eva Hevia & Sobi Asako
Nature Synthesis Published:25 March 2025
DOI:https://doi.org/10.1038/s44160-025-00771-1
Abstract
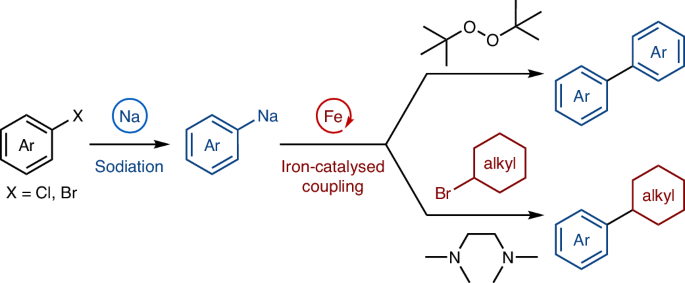
Sodium is one of the most abundant elements on Earth and a sustainable alternative to less sustainable metals such as lithium, which is becoming increasingly depleted and expensive. Traditionally, however, organosodium reagents have been considered highly reactive, engaging in uncontrollable reactions, and as a result, they have been scarcely used in organic synthesis, especially in combination with transition-metal catalysis. Here we report the use of organosodium compounds as C(sp2)–Na nucleophilic partners in iron-catalysed oxidative homocoupling and cross-coupling with alkyl halides. Mechanistic investigations based on the preparation and characterization of putative organoiron intermediates reveal that a bidentate additive coordinates both sodium and the iron centre, exerting control over the catalytic reactivity. This combination of two abundant and non-toxic metals, powered by molecular-level mechanistic understanding, is expected to open new avenues for the use of sustainable organometallic reagents in organic synthesis.