2023-05-03 ノースウェスタン大学
◆ノースウェスタン大学の研究者らが、キャプチャーされたCO2から得られる一酸化炭素を用いて酢酸を作り出す革新的な触媒を開発した。
◆この技術が、カーボンキャプチャー・ストレージの新たな関心を呼び起こす可能性がある。研究論文はNatureに掲載された。
<関連情報>
- https://news.northwestern.edu/stories/2023/05/catalyst-transforms-carbon-dioxide/
- https://www.nature.com/articles/s41586-023-05918-8
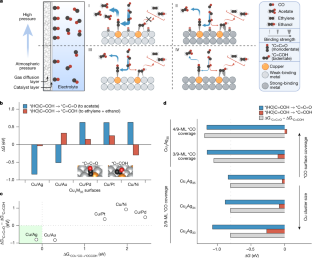
C2吸着剤の配向を抑制することで、COから酢酸への電解還元を可能にする Constrained C2 adsorbate orientation enables CO-to-acetate electroreduction
Jian Jin,Joshua Wicks,Qiuhong Min,Jun Li,Yongfeng Hu,Jingyuan Ma,Yu Wang,Zheng Jiang,Yi Xu,Ruihu Lu,Gangzheng Si,Panagiotis Papangelakis,Mohsen Shakouri,Qunfeng Xiao,Pengfei Ou,Xue Wang,Zhu Chen,Wei Zhang,Kesong Yu,Jiayang Song,Xiaohang Jiang,Peng Qiu,Yuanhao Lou,Dan Wu,Yu Mao,Adnan Ozden,Chundong Wang,Bao Yu Xia,Xiaobing Hu,Vinayak P. Dravid,Yun-Mui Yiu,Tsun-Kong Sham,Ziyun Wang,David Sinton,Liqiang Mai,Edward H. Sargent & Yuanjie Pang
Nature Published:03 May 2023
DOI:https://doi.org/10.1038/s41586-023-05918-8
Abstract
The carbon dioxide and carbon monoxide electroreduction reactions, when powered using low-carbon electricity, offer pathways to the decarbonization of chemical manufacture1,2. Copper (Cu) is relied on today for carbon–carbon coupling, in which it produces mixtures of more than ten C2+ chemicals3,4,5,6: a long-standing challenge lies in achieving selectivity to a single principal C2+ product7,8,9. Acetate is one such C2 compound on the path to the large but fossil-derived acetic acid market. Here we pursued dispersing a low concentration of Cu atoms in a host metal to favour the stabilization of ketenes10—chemical intermediates that are bound in monodentate fashion to the electrocatalyst. We synthesize Cu-in-Ag dilute (about 1 atomic per cent of Cu) alloy materials that we find to be highly selective for acetate electrosynthesis from CO at high *CO coverage, implemented at 10 atm pressure. Operando X-ray absorption spectroscopy indicates in situ-generated Cu clusters consisting of <4 atoms as active sites. We report a 12:1 ratio, an order of magnitude increase compared to the best previous reports, in the selectivity for acetate relative to all other products observed from the carbon monoxide electroreduction reaction. Combining catalyst design and reactor engineering, we achieve a CO-to-acetate Faradaic efficiency of 91% and report a Faradaic efficiency of 85% with an 820-h operating time. High selectivity benefits energy efficiency and downstream separation across all carbon-based electrochemical transformations, highlighting the importance of maximizing the Faradaic efficiency towards a single C2+ product11.