2025-03-03 カリフォルニア大学サンディエゴ校 (UCSD)
<関連情報>
- https://today.ucsd.edu/story/uc-san-diego-team-uncovers-mystery-of-waters-hidden-dual-phases
- https://www.nature.com/articles/s41567-024-02761-0
水中の液体-液体臨界点の位置に関する制約 Constraints on the location of the liquid–liquid critical point in water
F. Sciortino,Y. Zhai,S. L. Bore & F. Paesani
Nature Physics Published:03 February 2025
DOI:https://doi.org/10.1038/s41567-024-02761-0
Abstract
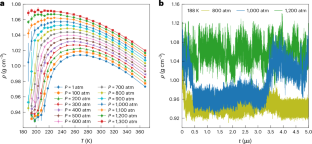
The fascinating hypothesis that supercooled water may segregate into two distinct liquid phases, each with unique structures and densities, was first posited in 1992. This idea, initially based on numerical analyses with the ST2 water-like empirical potential, challenged the conventional understanding of water’s phase behaviour at the time and has since intrigued the scientific community. Over the past three decades, advancements in computational modelling—particularly through the advent of data-driven many-body potentials rigorously derived from first principles and augmented by the efficiency of neural networks—have greatly enhanced the accuracy of molecular simulations, enabling the exploration of the phase behaviour of water with unprecedented realism. Our study leverages these computational advances to probe the elusive liquid–liquid transition in supercooled water. Microsecond-long simulations with chemical accuracy, conducted over several years, provide compelling evidence that water indeed exists in two discernibly distinct liquid states at low temperature and high pressure. By pinpointing a realistic estimate for the location of the liquid–liquid critical point at ~198 K and ~1,250 atm, our study not only advances the current understanding of water’s anomalous behaviour but also establishes a basis for experimental validation. Importantly, our simulations indicate that the liquid–liquid critical point falls within temperature and pressure ranges that could potentially be experimentally probed in water nanodroplets, opening up the possibility for direct measurements.