2024-12-05 パシフィック・ノースウェスト国立研究所(PNNL)
<関連情報>
- https://www.pnnl.gov/publications/crystallization-calcium-carbonate-dense-liquid-phase
- https://www.nature.com/articles/s41563-024-02025-5
炭酸カルシウム(bi)の濃密液相の形成、化学進化および凝固 Formation, chemical evolution and solidification of the dense liquid phase of calcium (bi)carbonate
Biao Jin,Ying Chen,Harley Pyles,Marcel D. Baer,Benjamin A. Legg,Zheming Wang,Nancy M. Washton,Karl T. Mueller,David Baker,Gregory K. Schenter,Christopher J. Mundy & James J. De Yoreo
Nature Materials Published:24 October 2024
DOI:https://doi.org/10.1038/s41563-024-02025-5
Abstract
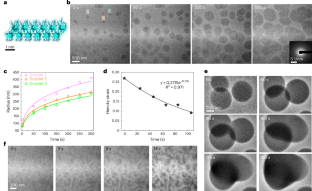
Metal carbonates, which are ubiquitous in the near-surface mineral record, are a major product of biomineralizing organisms and serve as important targets for capturing anthropogenic CO2 emissions. However, pathways of carbonate mineralization typically diverge from classical predictions due to the involvement of disordered precursors, such as the dense liquid phase (DLP), yet little is known about DLP formation or solidification processes. Using in situ methods we report that a highly hydrated bicarbonate DLP forms via liquid–liquid phase separation and transforms into hollow hydrated amorphous CaCO3 particles. Acidic proteins and polymers extend DLP lifetimes while leaving the pathway and chemistry unchanged. Molecular simulations suggest that the DLP forms via direct condensation of solvated Ca²+⋅(HCO3−)2 complexes that react due to proximity effects in the confined DLP droplets. Our findings provide insight into CaCO3 nucleation that is mediated by liquid–liquid phase separation, advancing the ability to direct carbonate mineralization and elucidating an often-proposed complex pathway of biomineralization.



