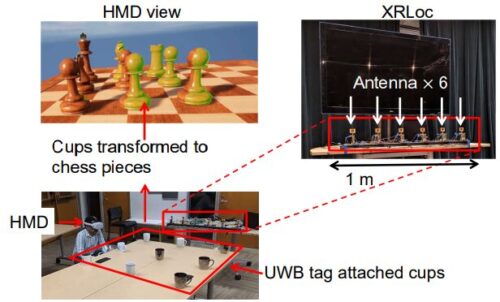
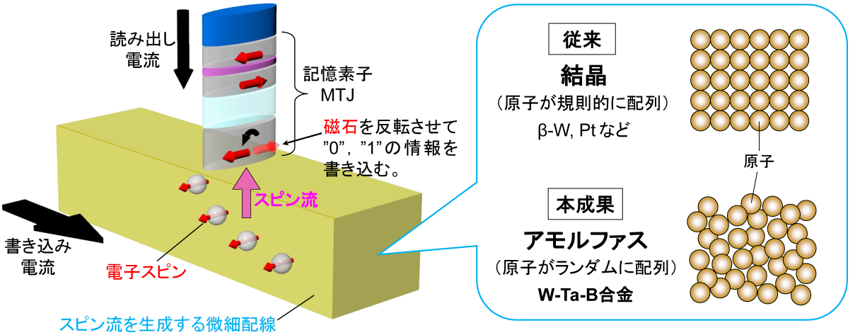
2023-12-19 韓国基礎科学研究院(IBS)
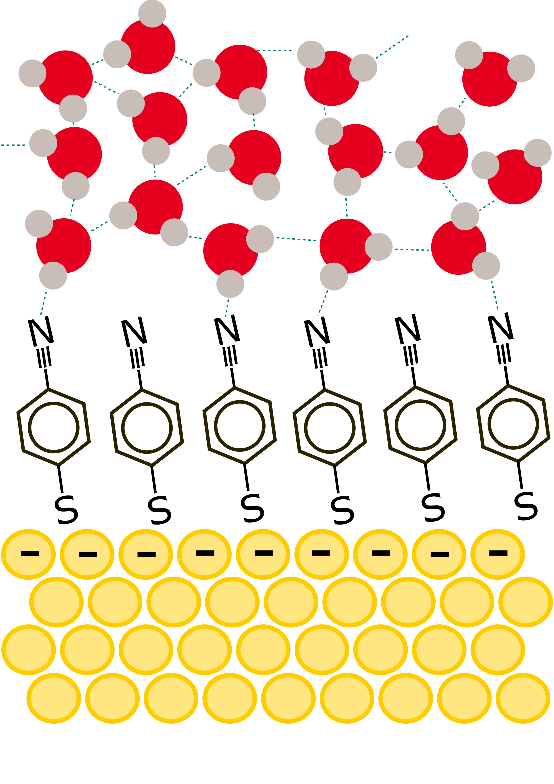
Figure 1. Schematic figure representing the organic molecules adsorbed on a gold surface and water molecules near the gold electrode.
◆通常の手法では金属原子の影響が大きく、それを軽減するために電極表面に特殊な有機分子をコーティングし、新しい分光学的技術とコンピュータシミュレーションを用いました。適用される電圧によって水分子の運動が初めて減速または加速することが観察され、これが電極表面での電気化学反応と水分子の動態との密接な関連性を示唆しています。
◆この成果は基本的な電化学プロセスの理解を深め、より効率的で持続可能なバッテリー技術の開発への道を拓くものと期待されています。
<関連情報>
- https://www.ibs.re.kr/cop/bbs/BBSMSTR_000000000738/selectBoardArticle.do
- https://www.pnas.org/doi/10.1073/pnas.2314998120
ニトリル官能基化電極への水の水素結合ダイナミクスが電圧によって変調することを2次元赤外分光法で明らかにした The hydrogen-bonding dynamics of water to a nitrile functionalized electrode is modulated by voltage according to 2D IR spectroscopy
Matthew J. Ryan, Nan Yang, Kijeong Kwac, Kiera B. Wilhelm, Benjamin K. Chi, Daniel J. Weix, Minhaeng Cho, and Martin T. Zanni
Proceedings of the National Academy of Sciences Published:December 21, 2023
DOI:https://doi.org/10.1073/pnas.2314998120
Significance
Critical reactions, such as transformations of small molecules, are catalyzed at electrochemical surfaces. Solvent reorientation influences reaction-free energy landscapes, yet little is known about how an applied electric field impacts solvent dynamics at a surface. Here, we use surface-enhanced two-dimensional infrared spectroscopy to measure the temporal evolution of spectroscopic features from a surface-bound nitrile label, whose CN stretching mode reports on the local hydrogen-bonding environment. We find that hydrogen bonds to nitrile groups break and form 2 to 3 times more slowly under positive potential compared to negative potential. These results reveal the dynamical consequences of an applied electric field at the electrode–water interface.
Abstract
We report the hydrogen-bonding dynamics of water to a nitrile-functionalized and plasmonic electrode surface as a function of applied voltage. The surface-enhanced two-dimensional infrared spectra exhibit hydrogen-bonded and non-hydrogen-bonded nitrile features in similar proportions, plus cross peaks between the two. Isotopic dilution experiments show that the cross peaks arise predominantly from chemical exchange between hydrogen-bonded and non-hydrogen-bonded nitriles. The chemical exchange rate depends upon voltage, with the hydrogen bond of the water to the nitriles breaking 2 to 3 times slower (>63 vs. 25 ps) under a positive as compared to a negative potential. Spectral diffusion created by hydrogen-bond fluctuations occurs on a ~1 ps timescale and is moderately potential-dependent. Timescales from molecular dynamics simulations agree qualitatively with the experiment and show that a negative voltage causes a small net displacement of water away from the surface. These results show that the voltage applied to an electrode can alter the timescales of solvent motion at its interface, which has implications for electrochemically driven reactions.