2023-05-25 ミュンヘン大学(LMU)
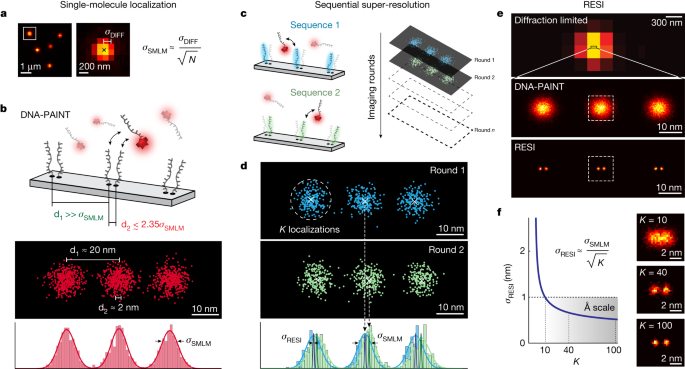
◆RESIは標準の蛍光顕微鏡で使用でき、細胞内構造や分子の解析に革命をもたらすと期待されています。さらに、DNA間のナノメートルスケールの距離を測定し、抗体の作用機序の解明にも成功しました。RESIは生物学研究において画期的な手法となり得るとされています。
<関連情報>
- https://www.lmu.de/en/newsroom/news-overview/news/beyond-the-limits-of-light.html
- https://www.nature.com/articles/s41586-023-05925-9
オングストローム分解能の蛍光顕微鏡観察 Ångström-resolution fluorescence microscopy
Susanne C. M. Reinhardt,Luciano A. Masullo,Isabelle Baudrexel,Philipp R. Steen,Rafal Kowalewski,Alexandra S. Eklund,Sebastian Strauss,Eduard M. Unterauer,Thomas Schlichthaerle,Maximilian T. Strauss,Christian Klein & Ralf Jungmann
Nature Published:24 May 2023
DOI:https://doi.org/10.1038/s41586-023-05925-9
Abstract
Fluorescence microscopy, with its molecular specificity, is one of the major characterization methods used in the life sciences to understand complex biological systems. Super-resolution approaches1,2,3,4,5,6 can achieve resolution in cells in the range of 15 to 20 nm, but interactions between individual biomolecules occur at length scales below 10 nm and characterization of intramolecular structure requires Ångström resolution. State-of-the-art super-resolution implementations7,8,9,10,11,12,13,14 have demonstrated spatial resolutions down to 5 nm and localization precisions of 1 nm under certain in vitro conditions. However, such resolutions do not directly translate to experiments in cells, and Ångström resolution has not been demonstrated to date. Here we introdue a DNA-barcoding method, resolution enhancement by sequential imaging (RESI), that improves the resolution of fluorescence microscopy down to the Ångström scale using off-the-shelf fluorescence microscopy hardware and reagents. By sequentially imaging sparse target subsets at moderate spatial resolutions of >15 nm, we demonstrate that single-protein resolution can be achieved for biomolecules in whole intact cells. Furthermore, we experimentally resolve the DNA backbone distance of single bases in DNA origami with Ångström resolution. We use our method in a proof-of-principle demonstration to map the molecular arrangement of the immunotherapy target CD20 in situ in untreated and drug-treated cells, which opens possibilities for assessing the molecular mechanisms of targeted immunotherapy. These observations demonstrate that, by enabling intramolecular imaging under ambient conditions in whole intact cells, RESI closes the gap between super-resolution microscopy and structural biology studies and thus delivers information key to understanding complex biological systems.