2023-05-10 オークリッジ国立研究所(ORNL)
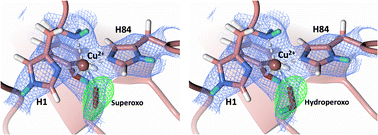
◆リチック多糖モノオキシゲナーゼと呼ばれる酵素(LPMOs)は、有機物を分解するのに優れているが、その作用機序は未だに明確になっていなかった。通常の酵素は炭素、窒素、水素、酸素で構成されているが、LPMOsには銅も含まれている。
◆オークリッジ国立研究所で中性子散乱を用いた研究により、LPMOsがバイオマスを分解する際に銅に酸素が結合する様子が既に明らかにされている。さらに、新たな研究で、アミノ酸が酸素分子にプロトンを提供して、この過程を推進していることが発見された。
◆オークリッジ国立研究所のFlora Meilleurは、「この新しい情報により、より効率的なLPMOsの改良バージョンを設計して試験できるようになり、セルロースエタノールの実現に役立ちます」と述べている。
<関連情報>
- https://www.ornl.gov/news/copper-key-more-efficient-biomass-breakdown
- https://pubs.rsc.org/en/content/articlelanding/2022/SC/D2SC05031E
溶菌性多糖類モノオキシゲナーゼの銅活性部位における活性化されたジオキシゲンの中間体の捕獲 Capture of activated dioxygen intermediates at the copper-active site of a lytic polysaccharide monooxygenase
Gabriela C. Schröder, William B. O’Dell, Simon P. Webb, Pratul K. Agarwal and Flora Meilleur
Chemical Science Published:02 Nov 2022
DOI:https://doi.org/10.1039/D2SC05031E
Abstract
Metalloproteins perform a diverse array of redox-related reactions facilitated by the increased chemical functionality afforded by their metallocofactors. Lytic polysaccharide monooxygenases (LPMOs) are a class of copper-dependent enzymes that are responsible for the breakdown of recalcitrant polysaccharides via oxidative cleavage at the glycosidic bond. The activated copper-oxygen intermediates and their mechanism of formation remains to be established. Neutron protein crystallography which permits direct visualization of protonation states was used to investigate the initial steps of oxygen activation directly following active site copper reduction in Neurospora crassa LPMO9D. Herein, we cryo-trap an activated dioxygen intermediate in a mixture of superoxo and hydroperoxo states, and we identify the conserved second coordination shell residue His157 as the proton donor. Density functional theory calculations indicate that both superoxo and hydroperoxo active site states are stable. The hydroperoxo formed is potentially an early LPMO catalytic reaction intermediate or the first step in the mechanism of hydrogen peroxide formation in the absence of substrate. We observe that the N-terminal amino group of the copper coordinating His1 remains doubly protonated directly following molecular oxygen reduction by copper. Aided by molecular dynamics and mining minima free energy calculations we establish that the conserved second-shell His161 in MtPMO3* displays conformational flexibility in solution and that this flexibility is also observed, though to a lesser extent, in His157 of NcLPMO9D. The imidazolate form of His157 observed in our structure following oxygen intermediate protonation can be attributed to abolished His157 flexibility due steric hindrance in the crystal as well as the solvent-occluded active site environment due to crystal packing. A neutron crystal structure of NcLPMO9D at low pH further supports occlusion of the active site since His157 remains singly protonated even at acidic conditions.