2023-02-27 ローレンスリバモア国立研究所(LLNL)
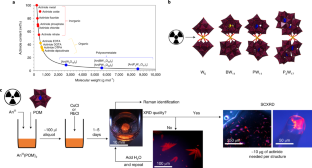
◆この新しい方法は、放射性物質の化学において、ポリオキソメタレートリガンド(POM)と呼ばれる分子を使用しています。POMの固有の性質により、わずか数マイクログラムで、ターゲットとなる放射性同位体を含む化合物を形成し、それらを結晶化して、様々な分光技術を用いて研究することができます。これにより、研究に必要な量を大幅に削減し、発見を急速に進めることができます。また、研究者に対する毒性リスクも低減されます。
◆この方法は、アクチニドと呼ばれる元素群(プルトニウムやウランなど)の化学的特性を研究することに適しています。これらは、地球上で生産されうる最も重い元素で、プルトニウムを超えると研究用同位体の入手性が急激に低下し、放射性が増加し、生産コストが劇的に上昇します。そのため、これらの元素に関する化学的特性については、あまり知られていません。
◆この研究の成果は、Nature ChemistryおよびInorganic Chemistryに掲載され、両雑誌の表紙に掲載されました。また、Nature誌でも紹介されました。今後、LLNLチームはこの研究を継続し、従来の方法では研究できなかった最も珍しいおよび毒性の高い元素に関する化学的知見を拡大することを目指しています。
<関連情報>
- https://www.llnl.gov/news/llnl-chemists-double-down-breakthrough-method-study-radioactive-materials
- https://www.nature.com/articles/s41557-022-01018-8
- https://pubs.acs.org/doi/10.1021/acs.inorgchem.2c04014
マイクログラムスケールの希少同位体化合物の合成、分離、特性評価のための配位子としてのポリオキソメタレート Polyoxometalates as ligands to synthesize, isolate and characterize compounds of rare isotopes on the microgram scale
Ian Colliard,Jonathan R. I. Lee,Christopher A. Colla,Harris E. Mason,April M. Sawvel,Mavrik Zavarin,May Nyman & Gauthier J.-P. Deblonde
Nature Chemistry Published:01 September 2022
DOI:https://doi.org/10.1038/s41557-022-01018-8
Abstract
The synthesis and study of radioactive compounds are both inherently limited by their toxicity, cost and isotope scarcity. Traditional methods using small inorganic or organic complexes typically require milligrams of sample—per attempt—which for some isotopes is equivalent to the world’s annual supply. Here we demonstrate that polyoxometalates (POMs) enable the facile formation, crystallization, handling and detailed characterization of metal–ligand complexes from microgram quantities owing to their high molecular weight and controllable solubility properties. Three curium–POM complexes were prepared, using just 1–10 μg per synthesis of the rare isotope 248Cm3+, and characterized by single-crystal X-ray diffraction, showing an eight-coordinated Cm3+ centre. Moreover, spectrophotometric, fluorescence, NMR and Raman analyses of several f-block element–POM complexes, including 243Am3+ and 248Cm3+, showed otherwise unnoticeable differences between their solution versus solid-state chemistry, and actinide versus lanthanide behaviour. This POM-driven strategy represents a viable path to isolate even rarer complexes, notably with actinium or transcalifornium elements.
溶液状態NMRによるポリオキソメタレートによる3価ランタニドおよびアクチニドの錯形成の対比 Contrasting Trivalent Lanthanide and Actinide Complexation by Polyoxometalates via Solution-State NMR
Christopher A. Colla, Ian Colliard, April M. Sawvel, May Nyman, Harris E. Mason, and Gauthier J.-P. Deblonde
Inorganic Chemistry Published:December 29, 2022
DOI:https://doi.org/10.1021/acs.inorgchem.2c04014

Abstract
Deciphering the solution chemistry and speciation of actinides is inherently difficult due to radioactivity, rarity, and cost constraints, especially for transplutonium elements. In this context, the development of new chelating platforms for actinides and associated spectroscopic techniques is particularly important. In this study, we investigate a relatively overlooked class of chelators for actinide binding, namely, polyoxometalates (POMs). We provide the first NMR measurements on americium–POM and curium–POM complexes, using one-dimensional (1D) 31P NMR, variable-temperature NMR, and spin-lattice relaxation time (T1) experiments. The proposed POM–NMR approach allows for the study of trivalent f-elements even when only microgram amounts are available and in phosphate-containing solutions where f-elements are typically insoluble. The solution-state speciation of trivalent americium, curium, plus multiple lanthanide ions (La3+, Nd3+, Sm3+, Eu3+, Yb3+, and Lu3+), in the presence of the model POM ligand PW11O397– was elucidated and revealed the concurrent formation of two stable complexes, [MIII(PW11O39)(H2O)x]4– and [MIII(PW11O39)2]11–. Interconversion reaction constants, reaction enthalpies, and reaction entropies were derived from the NMR data. The NMR results also provide experimental evidence of the weakly paramagnetic nature of the Am3+ and Cm3+ ions in solution. Furthermore, the study reveals a previously unnoticed periodicity break along the f-element series with the reversal of T1 relaxation times of the 1:1 and 1:2 complexes and the preferential formation of the long T1 species for the early lanthanides versus the short T1 species for the late lanthanides, americium, and curium. Given the broad variety of POM ligands that exist, with many of them containing NMR-active nuclei, the combined POM–NMR approach reported here opens a new avenue to investigate difficult-to-study elements such as heavy actinides and other radionuclides.