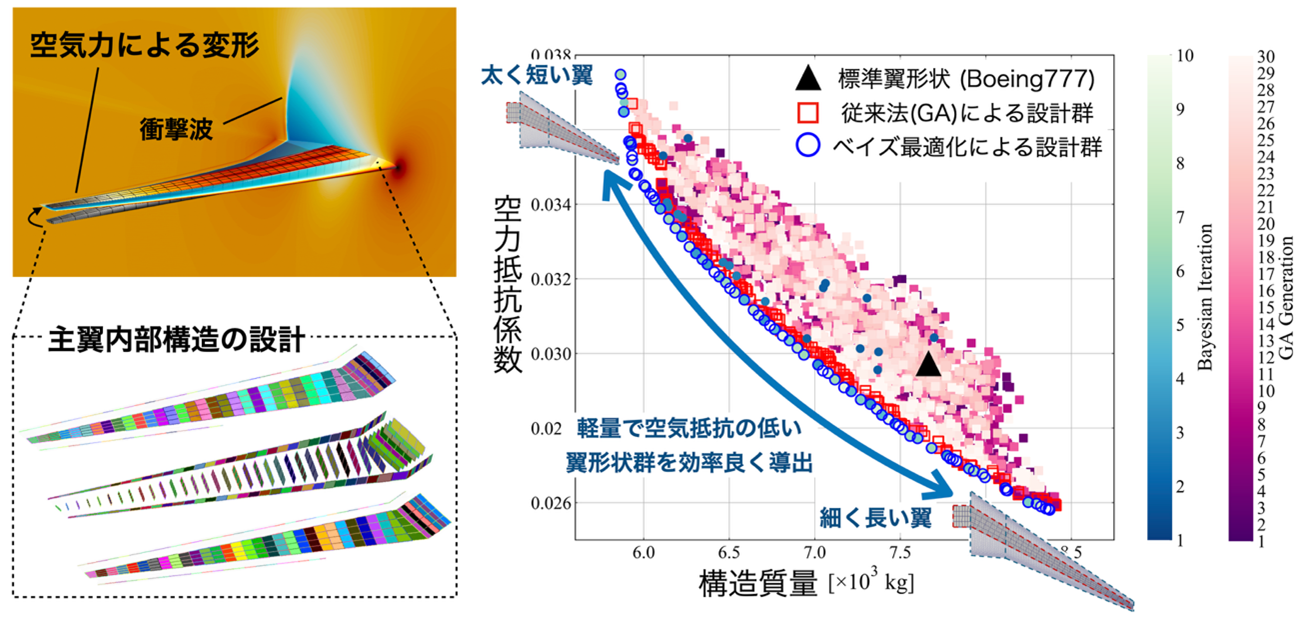
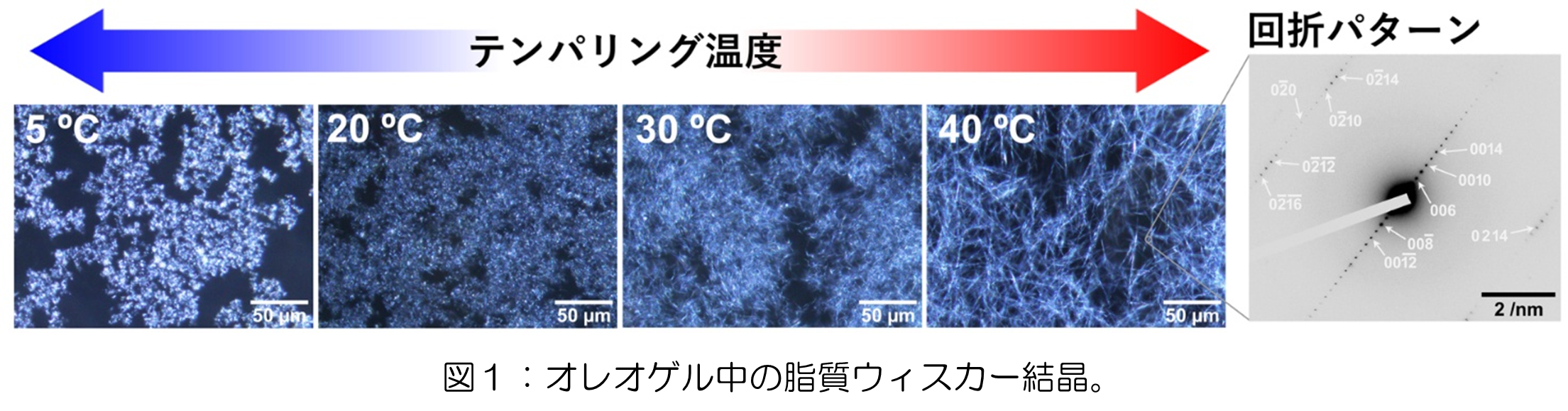
2026-02-19 東京科学大学
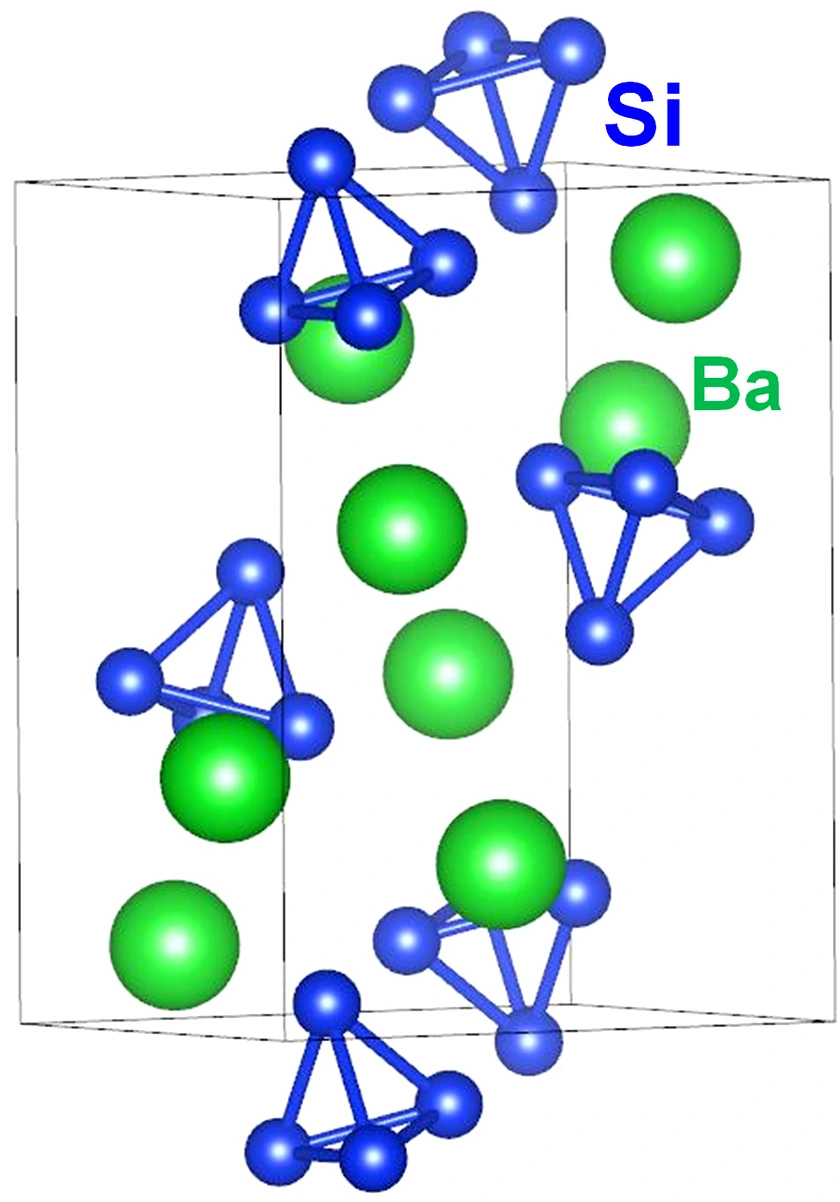
図1. BaSi2の結晶構造
<関連情報>
三元遷移金属窒化物中間体の形成によるアンモニア分解の促進:Ni(Co)担持BaSi2 Ammonia Decomposition Promoted by the Formation of Ternary Transition Metal Nitride Intermediates: Ni (Co)-Loaded BaSi2
Qing Guo,Shiyao Wang,Jiang Li,Yihao Jiang,Zhujun Zhang,Masato Sasase,Hideo Hosono,and Masaaki Kitano
Journal of the American Chemical Society
DOI:https://doi.org/10.1021/jacs.5c16307
Abstract
Catalytic ammonia decomposition for hydrogen release at reduced temperatures is a critical component of the hydrogen energy roadmap. However, conventional nonprecious transition metal (TM) catalysts, such as Ni and Co, typically suffer from a high energy barrier in the N–N coupling step. In this study, we report that a stable Zintl phase silicide of BaSi2 functions as an efficient support for Ni and Co catalysts in ammonia decomposition via the formation of TM–nitrogen–barium intermediates at the TM-BaSi2 interface. Characterizations using kinetic studies, X-ray photoelectron spectroscopy (XPS), and density functional theory calculations suggest that electron transfer occurs from the interfacial low-valence barium to nitrogen atoms bonded to TM at the TM-BaSi2 interface, which leads to the formation of TM-nitrogen–barium intermediates and in turn lowers the energy barrier for the rate-determining N–N coupling step. This promotion enables Ni- or Co-loaded BaSi2 catalysts to significantly outperform conventional nonprecious metal catalysts and become comparable to the Ru-based catalysts. These results offer a new perspective for the future design of nonprecious metal catalysts for ammonia decomposition at reduced temperature.