2025-11-24 カリフォルニア大学バークレー校(UC Berkeley)
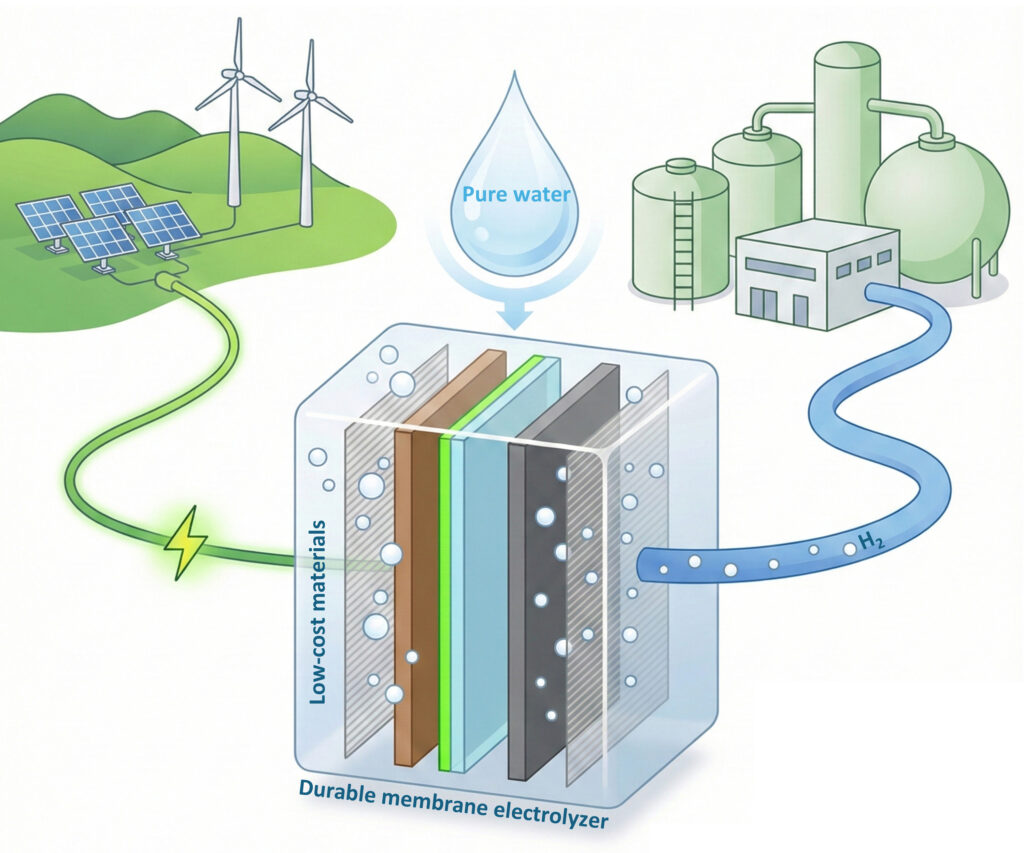
Low-cost, durable electrolyzers (center) could use sustainable power from wind and solar to turn pure water into hydrogen gas that can fuel industrial plants as well as heavy vehicles.Yang Zhao/UC Berkeley
<関連情報>
- https://news.berkeley.edu/2025/11/24/how-uc-berkeley-is-improving-the-affordability-of-hydrogen-fuel/
- https://www.science.org/doi/10.1126/science.adw7100
界面工学による耐久性のある純水供給型陰イオン交換膜電解装置 Durable, pure water–fed, anion-exchange membrane electrolyzers through interphase engineering
Shujin Hou, Archana Sekar, Yang Zhao, Minkyoung Kwak, […] , and Shannon W. Boettcher
Science Published:16 Oct 2025
DOI:https://doi.org/10.1126/science.adw7100
Editor’s summary
Anion-exchange membrane water electrolyzers represent one of the most economical methods for producing green hydrogen. However, their limited durability, particularly during operation with pure water, remains a major obstacle to widespread adoption. Hou et al. investigated ionomer binder design in greater depth and demonstrated that appropriately introduced inorganic additives can help to prevent oxidative degradation of the ionomer while preserving excellent mechanical integrity and ionic conductivity, ultimately extending device lifetime (see the Perspective by Kim). The enhanced stabilization originates from cross-linking between the additive and the ionomer and is broadly applicable across combinations of catalysts, additives, and ionomers. —Jack Huang
Abstract
Anion-exchange membrane water electrolyzers (AEMWEs) promise scalable, low-cost hydrogen production but are limited by the electrochemical instability of their anode ionomers. We report interphase engineering using inorganic-containing molecular additives that coassemble with ionomer, enabling pure water–fed AEMWEs to operate with a degradation rate <0.5 millivolt per hour at 2.0 amperes per square centimeter and 70°C—a >20-fold durability improvement. Analysis of different additives and ionomers shows that the stabilization mechanism involves cross-links between metal oxo/hydroxo oligomers and ionomers. Under operation, the inorganic additive enriches, forming an interphase near the water-oxidation catalyst that passivates the anode ionomer against continuous degradation while maintaining mechanical integrity and hydroxide conductivity. This additive-based interphase-engineering strategy provides a path to durable AEMWEs that operate without supporting electrolytes and is adaptable across diverse catalysts and ionomers for electrochemical technologies.



