2025-07-25 パシフィック・ノースウェスト国立研究所(PNNL)
ChatGPT:
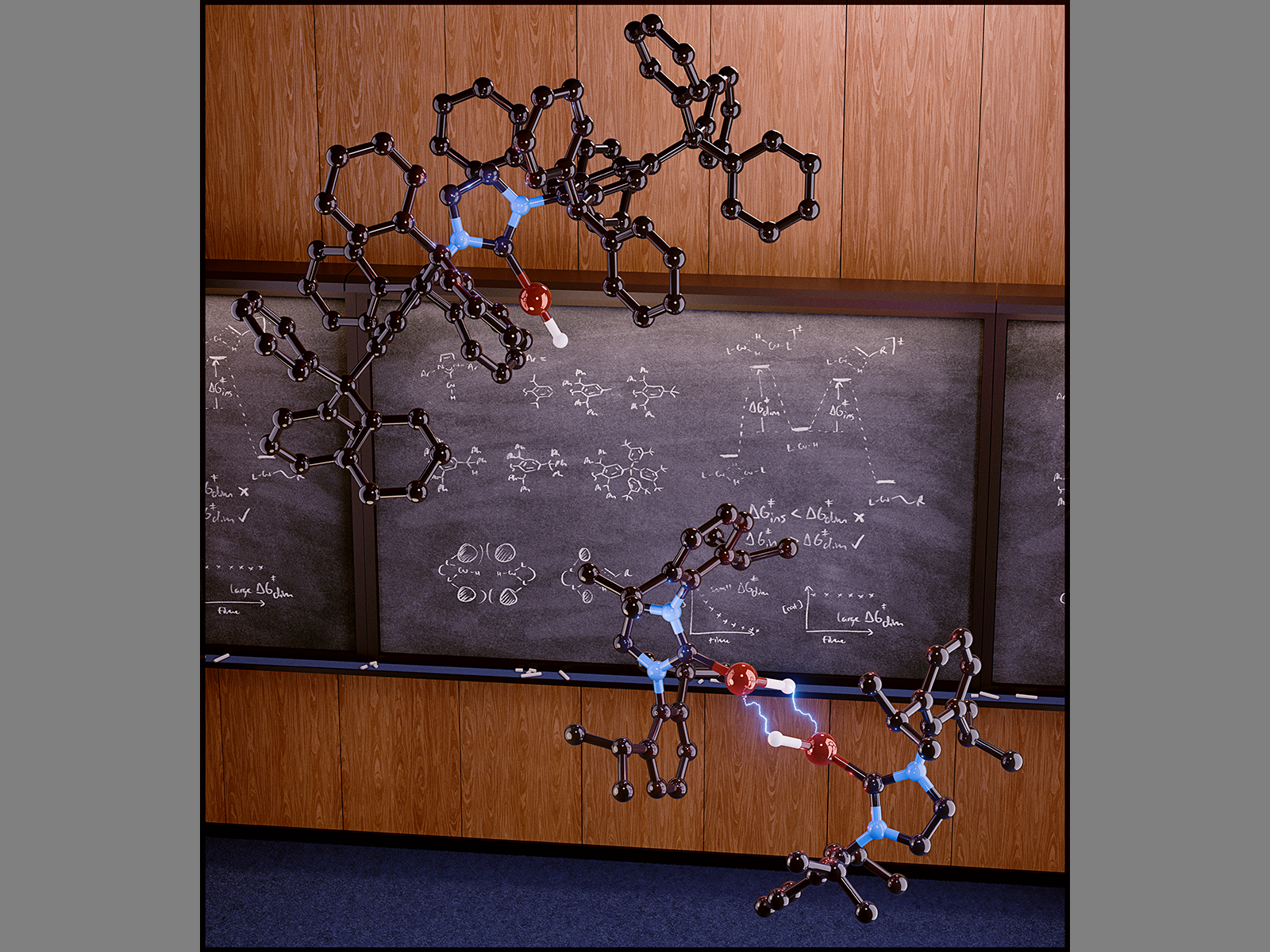
Mapping copper hydride aggregation mechanisms and structurally characterizing copper hydride clusters enabled identification of key features for consideration in ligand design. (Image by David Ryan | Pacific Northwest National Laboratory)
<関連情報>
- https://www.pnnl.gov/publications/analyzing-stability-and-aggregation-copper-hydride-monomers
- https://pubs.acs.org/doi/full/10.1021/jacs.4c17955
分子銅水素化物触媒のメカニズム的洞察:CuHモノマーの凝集に対する動的安定性は、触媒性能の重要なパラメーターである Mechanistic Insights into Molecular Copper Hydride Catalysis: the Kinetic Stability of CuH Monomers toward Aggregation is a Critical Parameter for Catalyst Performance
David E. Ryan,Jack T. Fuller III,Evan A. Patrick,Jeremy D. Erickson,Amy L. Speelman,Timothy G. Carroll,Gregory K. Schenter,Bojana Ginovska,Simone Raugei,R. Morris Bullock,and Ba L. Tran
Journal of the American Chemical Society Published: March 31, 2025
DOI:https://doi.org/10.1021/jacs.4c17955
Abstract
The activity of molecular copper hydride (CuH) complexes toward the selective insertion of unsaturated hydrocarbons under mild conditions has contributed significantly to versatile methodologies for upgrading these feedstocks. However, these catalysts are particularly susceptible to deleterious aggregation, leading to the depletion of active CuH species. Little is known about the mechanisms of CuH aggregation, how it influences overall catalyst performance, and how it can be controlled. We address these challenges with mechanistic studies on a model reaction of unactivated alkene hydroboration catalyzed by (IPr*CPh3)CuH (LCuH). We report a comprehensive mechanistic investigation of this system, identifying an aggregation pathway that continuously depletes catalytically active LCuH to form inactive CuH clusters during turnover. Deactivation of LCuH is controlled primarily by the competition between the kinetics of the initial LCuH dimerization step and that of alkene insertion into LCuH. We therefore propose that a comprehensive understanding of CuH catalyst performance must account for the kinetics of the initial LCuH dimerization step, revising a previously explored thermodynamic understanding of CuH aggregation, where the concentration of active species is controlled by equilibria established between CuH clusters and monomers. With a series of (NHC)CuH congeners (NHC = N-heterocyclic carbene), we demonstrate that ostensibly minor structural modifications to the ligand peripheries can drastically affect the LCuH dimerization kinetics, while maintaining reactivity toward on-cycle alkene insertion. We employed a computational approach based on molecular dynamics simulations to provide an in-depth understanding of how specific structural ligand modifications can substantially increase the kinetic stability of monomeric CuH catalysts. Our combined experimental and computational studies suggest strategies for rational ligand design that can be broadly applied to molecular catalyst systems that are susceptible to deactivation via aggregation pathways.