2023-12-05 マサチューセッツ工科大学(MIT)
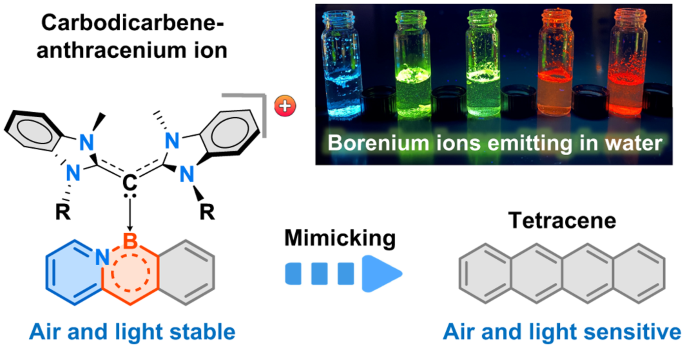
◆これまでのアセンは分解しやすく、安定性が課題でしたが、MITの新しいアプローチにより、赤、オレンジ、黄、緑、青の色を発光するアセンを合成できました。また、これらのアセンは水と空気に対しても安定しており、医学的な用途にも適しています。今後は、より安定性と光量子効率を持つ派生体の開発が期待され、太陽電池やLEDなどへの応用が進む見通しです。
<関連情報>
- https://news.mit.edu/2023/chemists-create-organic-molecules-rainbow-colors-1205
- https://www.nature.com/articles/s41557-023-01381-0
空気中でも光でも安定な発光性カルボジカルベン-アザボラアセニウムイオン Air- and photo-stable luminescent carbodicarbene-azaboraacenium ions
Chun-Lin Deng,Akachukwu D. Obi,Bi Youan E. Tra,Samir Kumar Sarkar,Diane A. Dickie & Robert J. Gilliard Jr
Nature Chemistry Published:05 December 2023
DOI:https://doi.org/10.1038/s41557-023-01381-0
Abstract
Substitution of a C=C bond by an isoelectronic B–N bond is a well-established strategy to alter the electronic structure and stability of acenes. BN-substituted acenes that possess narrow energy gaps have attractive optoelectronic properties. However, they are susceptible to air and/or light. Here we present the design, synthesis and molecular structures of fully π-conjugated cationic BN-doped acenes stabilized by carbodicarbene ligands. They are luminescent in the solution and solid states and show high air and moisture stability. Compared with their neutral BN-substituted counterparts as well as the parent all-carbon acenes, these species display improved quantum yields and small optical gaps. The electronic structures of the azabora-anthracene and azabora-tetracene cations resemble higher-order acenes while possessing high photo-oxidative resistance. Investigations using density functional theory suggest that the stability and photo-physics of these conjugated systems may be ascribed to their cationic nature and the electronic properties of the carbodicarbene.