2025-10-20 北海道大学,名古屋大学,自然科学研究機構,北海道立総合研究機構
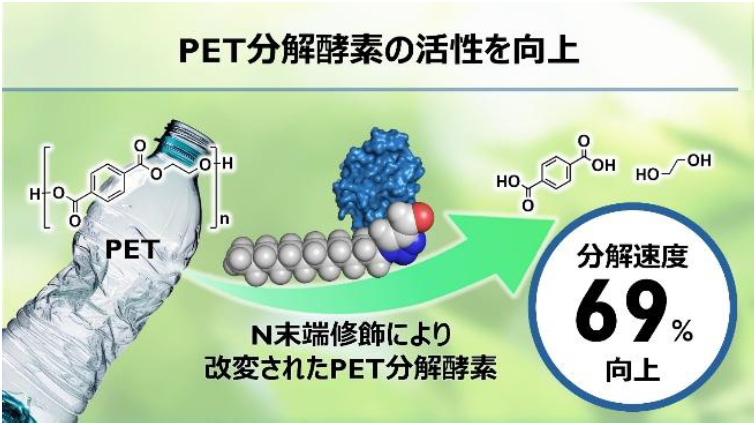
PET分解酵素のN末端に疎水性アルキル鎖を連結した改変酵素は、PET加水分解の活性を大幅に向上。
<関連情報>
- https://www.hokudai.ac.jp/news/2025/10/pet69n.html
- https://www.hokudai.ac.jp/news/pdf/251020_pr2.pdf
- https://pubs.acs.org/doi/10.1021/acssuschemeng.5c05212
N末端に疎水性部位を連結したクチナーゼによるポリエチレンテレフタレートの吸着促進と酵素分解の向上 Enhanced Adsorption and Enzymatic Hydrolysis of Polyethylene Terephthalate by Cutinase with an N-Terminal Hydrophobic Tether
Md Sadikur Rahman Shuvo,Doris Ribitsch,Georg M. Gübitz,Shuichiro Seno,Takayuki Uchihashi,and Akira Onoda
ACS Sustainable Chemistry & Engineering Published: October 5, 2025
DOI:https://doi.org/10.1021/acssuschemeng.5c05212
Abstract
The environmental challenges presented by plastic waste, particularly poly(ethylene terephthalate) (PET), necessitate innovative biodegradation strategies. The cutinase from Thermobifida cellulosilytica, Thc_Cut1 (Cut), was site-specifically conjugated with alkyl tethers of varying lengths (C3, C6, C9) through 1H-1,2,3-triazole-4-carbaldehyde (TA4C) derivatives. These conjugations were designed to enhance affinity for PET by adjusting the enzyme’s hydrophobicity. The enzyme kinetic parameters of both conjugated and unconjugated cutinases revealed that the modifications have a minimal impact on catalytic activity. However, a significant improvement in the PET hydrolysis efficiency was observed. Specifically, hexyl and nonyl TA4C-containing cutinase displayed notable increases in terephthalic acid (TPA) release, exceeding the performance of unconjugated cutinase by 65% and 69%, respectively. Scanning electron microscopy and water contact angle measurements confirmed the enhanced erosion and hydrophilicity of the PET surface following the enzyme treatment. Increased enzyme adsorption on the PET surface for C6–Cut and C9–Cut was validated by X-ray photoelectron spectroscopy. Moreover, high-speed atomic force microscopy demonstrated faster and more stable adsorption of C6–Cut and C9–Cut on PET surfaces compared with the slower adsorption of unconjugated cutinase. Additionally, molecular dynamics simulations indicate a higher affinity of conjugated cutinase for PET film. These results suggest that conjugating an alkyl tether to the N-terminus strengthens the interaction between cutinase and PET, improving hydrolysis.



