2025-09-18 東北大学
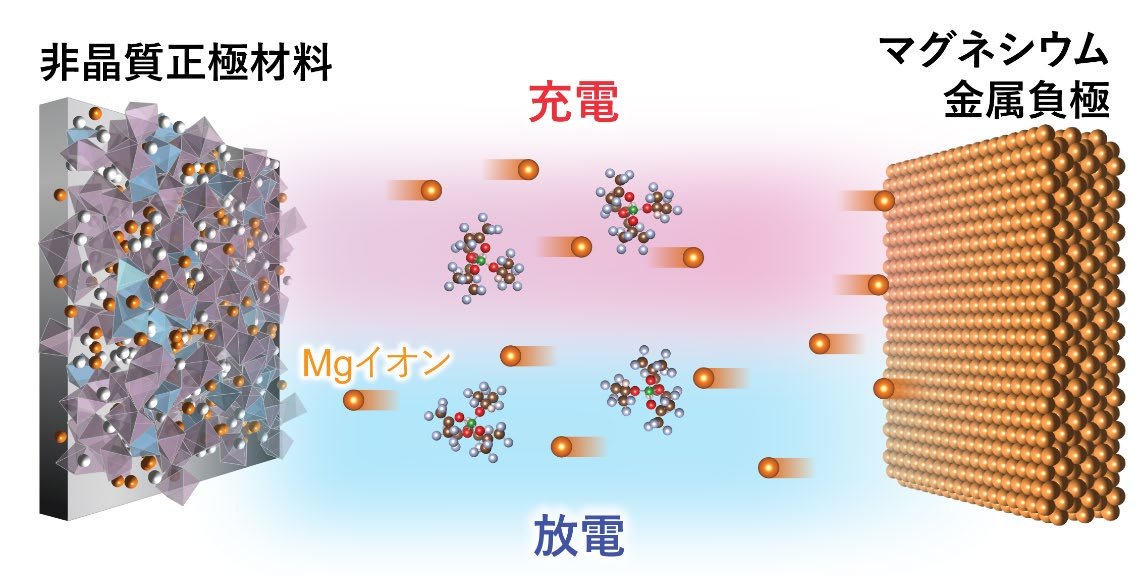
図1. 本研究のMg蓄電池の作動原理の模式図。充電では非晶質正極材料からMgイオンが脱離し、電解液中を移動するとともに、Mg金属負極上では電析反応が進行する。一方放電では、Mg金属負極からMgイオンが溶解し、電解液中のMgイオンが正極に挿入される。正極は原子配列が周期的ではない、非晶質の酸化物材料が用いられる。
<関連情報>
- https://www.tohoku.ac.jp/japanese/2025/09/press20250918-01-mg.html
- https://www.tohoku.ac.jp/japanese/newimg/pressimg/tohokuuniv-press20250918_01web_.pdf
- https://www.nature.com/articles/s43246-025-00921-0
常温充電式マグネシウム電池を実現する非晶質酸化物カソード Amorphous oxide cathode enabling room-temperature rechargeable magnesium batteries
Tomoya Kawaguchi,Hikari Sakurai,Shusuke Fukui,Xiatong Ye,Hongyi Li,Toshihiko Mandai,Norihiko L. Okamoto & Tetsu Ichitsubo
Communications Materials Published:17 September 2025
DOI:https://doi.org/10.1038/s43246-025-00921-0
Abstract
Rechargeable magnesium batteries (RMBs) have faced challenges in utilizing oxide cathodes due to the inherently sluggish Mg diffusion and poor compatibility with electrolytes, despite the high redox potential. Herein, we present a prototype RMB that is operational at room temperature, consisting of a nanoparticulate amorphous oxide cathode, fluorinated alkoxyborate (Mg[B(HFIP)4]2) as the electrolyte, and a Mg metal anode. The amorphous MgxTi1/9Mo2/9O cathode contains a considerable free volume formed by ion exchange between monovalent and divalent cations, facilitating Mg diffusion and eventually realizing reversible Mg insertion/extraction at room temperature. The reasonable compatibility of the present cathode with the electrolyte enables full cell operation with a Mg metal anode, and various analyses have demonstrated that Mg intercalation is responsible for the battery performance. The discharging capacity is ~150 mAh g−1, and 70 mAh g−1 is maintained after 200 cycles. These findings demonstrate the feasibility of RMBs with oxide cathodes that are operational at room temperature.