2025-07-22 ペンシルベニア州立大学(Penn State)
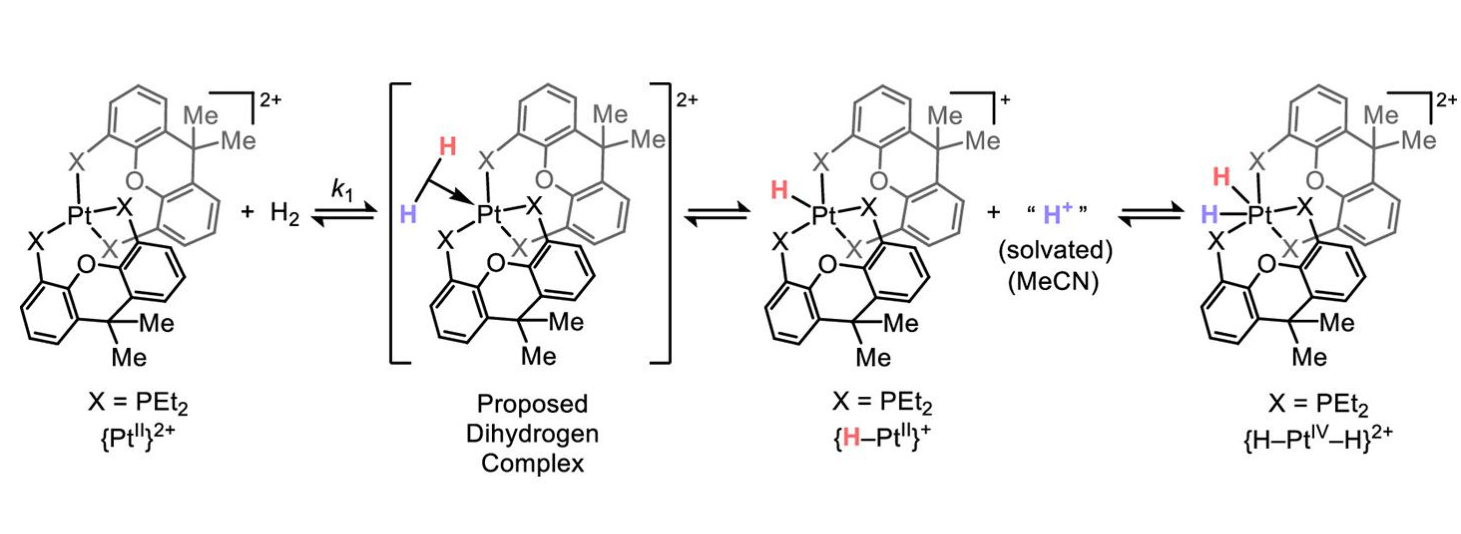
New play in transition metal chemistry playbook revealed. New research shows that net oxidative addition can occur via a different order of events in which the transitiion metal complex accepts electrons from an organic substrate (H2). Credit: Provided by the researchers. All Rights Reserved.
<関連情報>
- https://www.psu.edu/news/eberly-college-science/story/new-play-chemical-reaction-playbook-uncovered
- https://pubs.acs.org/doi/10.1021/jacs.5c07140
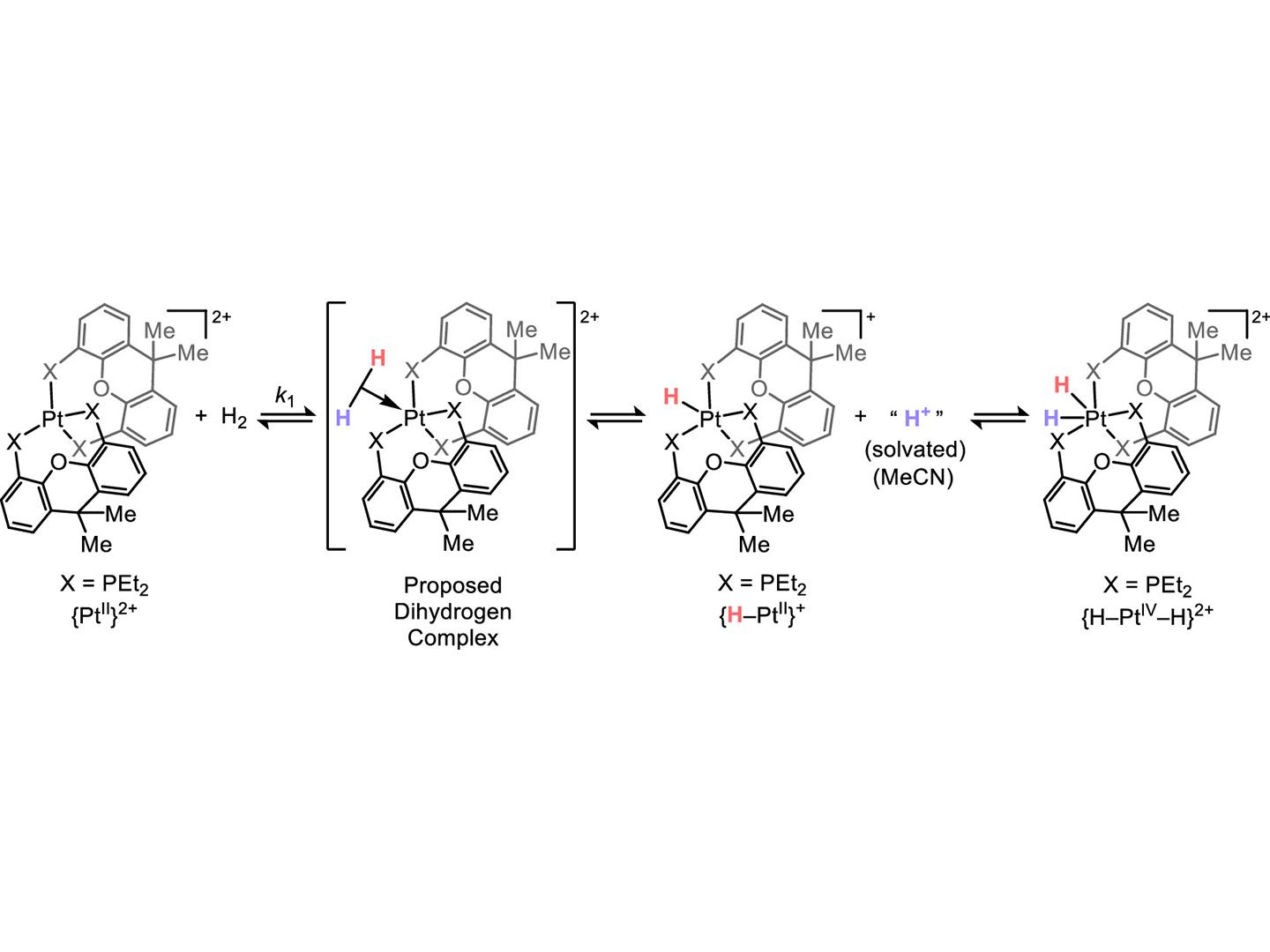
ヘテロリシスとプロトン性リバウンドによる{MII}2+(M = Pd, Pt)へのH2の正味の酸化的付加 Net Oxidative Addition of H2 to {MII}2+ (M = Pd, Pt) by Heterolysis and Protic Rebound
Nisha Rao,Jonathan L. Kuo
Journal of the American Chemical Society Published: June 23, 2025
DOI:https://doi.org/10.1021/jacs.5c07140
Abstract
Electrophilic transition metal complexes like {MII(EtXantphos)2}2+ (MII = PdII, PtII) heterolyze H2 into a hydride-associated electrophile {H–MII(EtXantphos)2}+ and a proton, which typically associates to an added base (or basic ligand). For {H–MII(EtXantphos)2}+, the metal can be the most basic site in the system, which results in a product that is indistinguishable from oxidative addition {(H)2MIV(EtXantphos)2}2+. By considering the kinetics and thermodynamics of each elementary step – initial heterolysis, followed by a subsequent return of the heterolyzed proton – we suggest that oxidative addition products may be underrepresented tautomers in heterolytic pathways. The gained understanding was used to characterize the first (di)hydride of PdIV, generated by formal oxidative addition of H2 to PdII.