2024-06-19 ノースカロライナ州立大学(NCState)
<関連情報>
溶媒で強化されたガラス状ゲル Glassy gels toughened by solvent
Meixiang Wang,Xun Xiao,Salma Siddika,Mohammad Shamsi,Ethan Frey,Wen Qian,Wubin Bai,Brendan T. O’Connor & Michael D. Dickey
Nature Published:19 June 2024
DOI:https://doi.org/10.1038/s41586-024-07564-0
Abstract
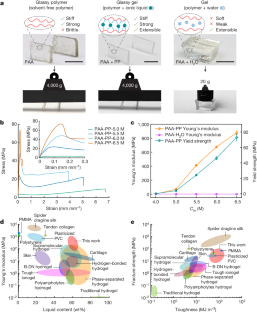
Glassy polymers are generally stiff and strong yet have limited extensibility1. By swelling with solvent, glassy polymers can become gels that are soft and weak yet have enhanced extensibility1,2,3. The marked changes in properties arise from the solvent increasing free volume between chains while weakening polymer–polymer interactions. Here we show that solvating polar polymers with ionic liquids (that is, ionogels4,5) at appropriate concentrations can produce a unique class of materials called glassy gels with desirable properties of both glasses and gels. The ionic liquid increases free volume and therefore extensibility despite the absence of conventional solvent (for example, water). Yet, the ionic liquid forms strong and abundant non-covalent crosslinks between polymer chains to render a stiff, tough, glassy, and homogeneous network (that is, no phase separation)6, at room temperature. Despite being more than 54 wt% liquid, the glassy gels exhibit enormous fracture strength (42 MPa), toughness (110 MJ m−3), yield strength (73 MPa) and Young’s modulus (1 GPa). These values are similar to those of thermoplastics such as polyethylene, yet unlike thermoplastics, the glassy gels can be deformed up to 670% strain with full and rapid recovery on heating. These transparent materials form by a one-step polymerization and have impressive adhesive, self-healing and shape-memory properties.