2024-05-14 シンガポール国立大学(NUS)
<関連情報>
- https://news.nus.edu.sg/new-technique-to-transform-waste-carbon-dioxide-into-high-value-chemicals/
- https://www.nature.com/articles/s41467-024-46175-1
- https://www.nature.com/articles/s41467-024-45527-1
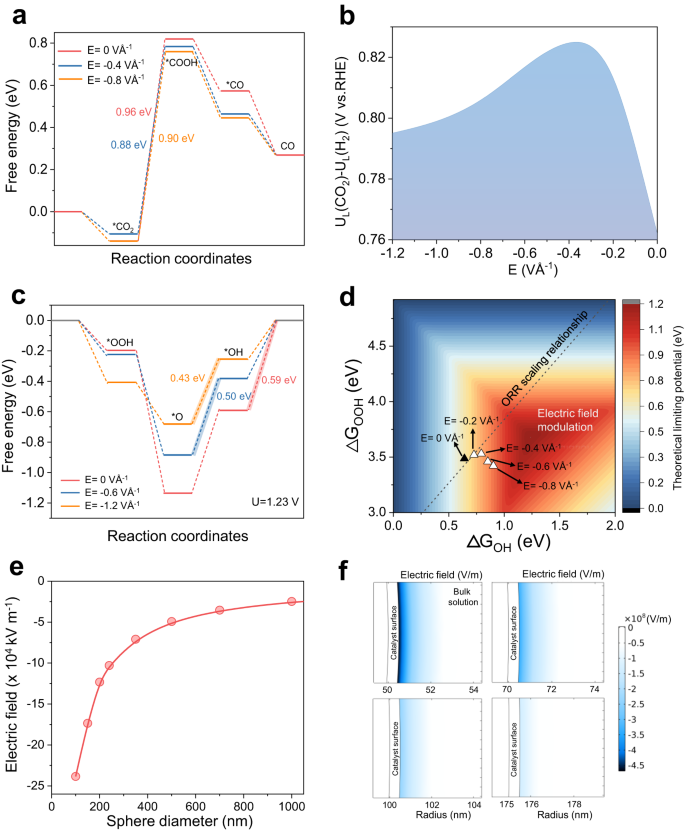
ナノ曲率誘起電界効果で単一原子電極触媒の活性制御が可能に Nanocurvature-induced field effects enable control over the activity of single-atom electrocatalysts
Meng Wang,Bingqing Wang,Jiguang Zhang,Shibo Xi,Ning Ling,Ziyu Mi,Qin Yang,Mingsheng Zhang,Wan Ru Leow,Jia Zhang & Yanwei Lum
Nature Communications Published:26 February 2024
DOI:https://doi.org/10.1038/s41467-024-46175-1
Abstract
Tuning interfacial electric fields provides a powerful means to control electrocatalyst activity. Importantly, electric fields can modify adsorbate binding energies based on their polarizability and dipole moment, and hence operate independently of scaling relations that fundamentally limit performance. However, implementation of such a strategy remains challenging because typical methods modify the electric field non-uniformly and affects only a minority of active sites. Here we discover that uniformly tunable electric field modulation can be achieved using a model system of single-atom catalysts (SACs). These consist of M-N4 active sites hosted on a series of spherical carbon supports with varying degrees of nanocurvature. Using in-situ Raman spectroscopy with a Stark shift reporter, we demonstrate that a larger nanocurvature induces a stronger electric field. We show that this strategy is effective over a broad range of SAC systems and electrocatalytic reactions. For instance, Ni SACs with optimized nanocurvature achieved a high CO partial current density of ~400 mA cm−2 at >99% Faradaic efficiency for CO2 reduction in acidic media.
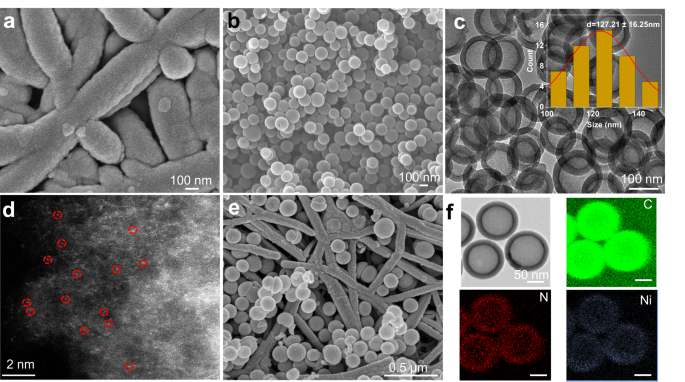
酸性媒体により、模擬排ガスから多炭素生成物の酸素耐性電解合成が可能に Acidic media enables oxygen-tolerant electrosynthesis of multicarbon products from simulated flue gas
Meng Wang,Bingqing Wang,Jiguang Zhang,Shibo Xi,Ning Ling,Ziyu Mi,Qin Yang,Mingsheng Zhang,Wan Ru Leow,Jia Zhang & Yanwei Lum
Nature Communications Published:09 February 2024
DOI:https://doi.org/10.1038/s41467-024-45527-1
Abstract
Renewable electricity powered electrochemical CO2 reduction (CO2R) offers a valuable method to close the carbon cycle and reduce our overreliance on fossil fuels. However, high purity CO2 is usually required as feedstock, which potentially decreases the feasibility and economic viability of the process. Direct conversion of flue gas is an attractive option but is challenging due to the low CO2 concentration and the presence of O2 impurities. As a result, up to 99% of the applied current can be lost towards the undesired oxygen reduction reaction (ORR). Here, we show that acidic electrolyte can significantly suppress ORR on Cu, enabling generation of multicarbon products from simulated flue gas. Using a composite Cu and carbon supported single-atom Ni tandem electrocatalyst, we achieved a multicarbon Faradaic efficiency of 46.5% at 200 mA cm-2, which is ~20 times higher than bare Cu under alkaline conditions. We also demonstrate stable performance for 24 h with a multicarbon product full-cell energy efficiency of 14.6%. Strikingly, this result is comparable to previously reported acidic CO2R systems using pure CO2. Our findings demonstrate a potential pathway towards designing efficient electrolyzers for direct conversion of flue gas to value-added chemicals and fuels.